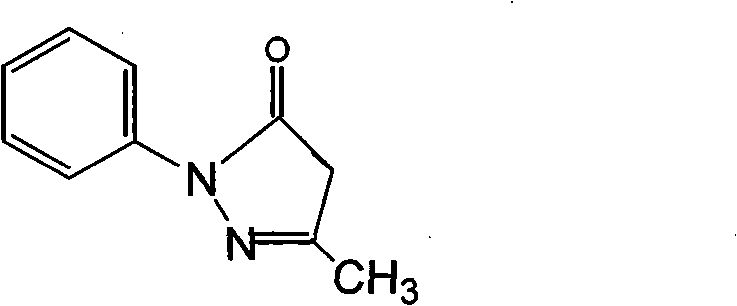
Edaravone injection and preparation process thereof
A technology of edaravone and preparation process, applied in the field of edaravone injection preparation and preparation process, can solve the problems of easy oxidation, poor stability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
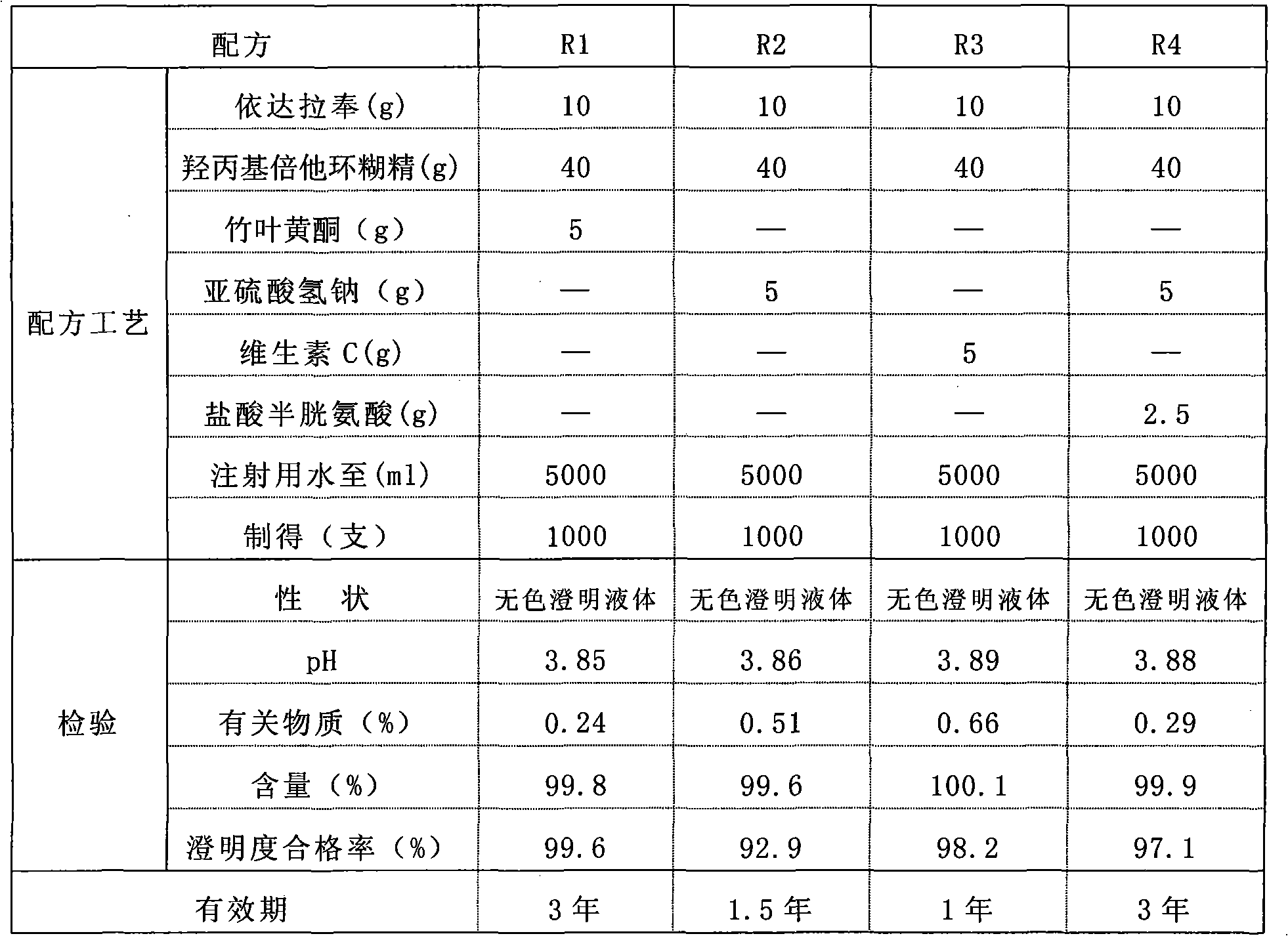
[0054] Formula (specification: 5ml: 10mg)
[0055] Raw materials
Dosage
effect
Specification
Edaravone
10g
Main drug
Medicinal
Hydroxypropyl Beta Cyclodextrin
40g
Co-solvent
Medicinal
Bamboo leaf flavonoids
5g
Medicinal
Add water for injection to
5000ml
Injection
Residual oxygen 1.8%
prevent oxidation
High purity nitrogen
[0056] Add the prescribed amount of Edaravone, hydroxypropyl beta cyclodextrin, and bamboo leaf flavonoids into water for injection, stir until completely dissolved, add activated carbon for needles, and oxidize with 0.1mol / L hydrochloric acid and / or hydrogen after removing the carbon Adjust the pH with sodium solution, fill it with nitrogen, seal it in an ampoule, and sterilize it by autoclaving at 115°C for 30 minutes.
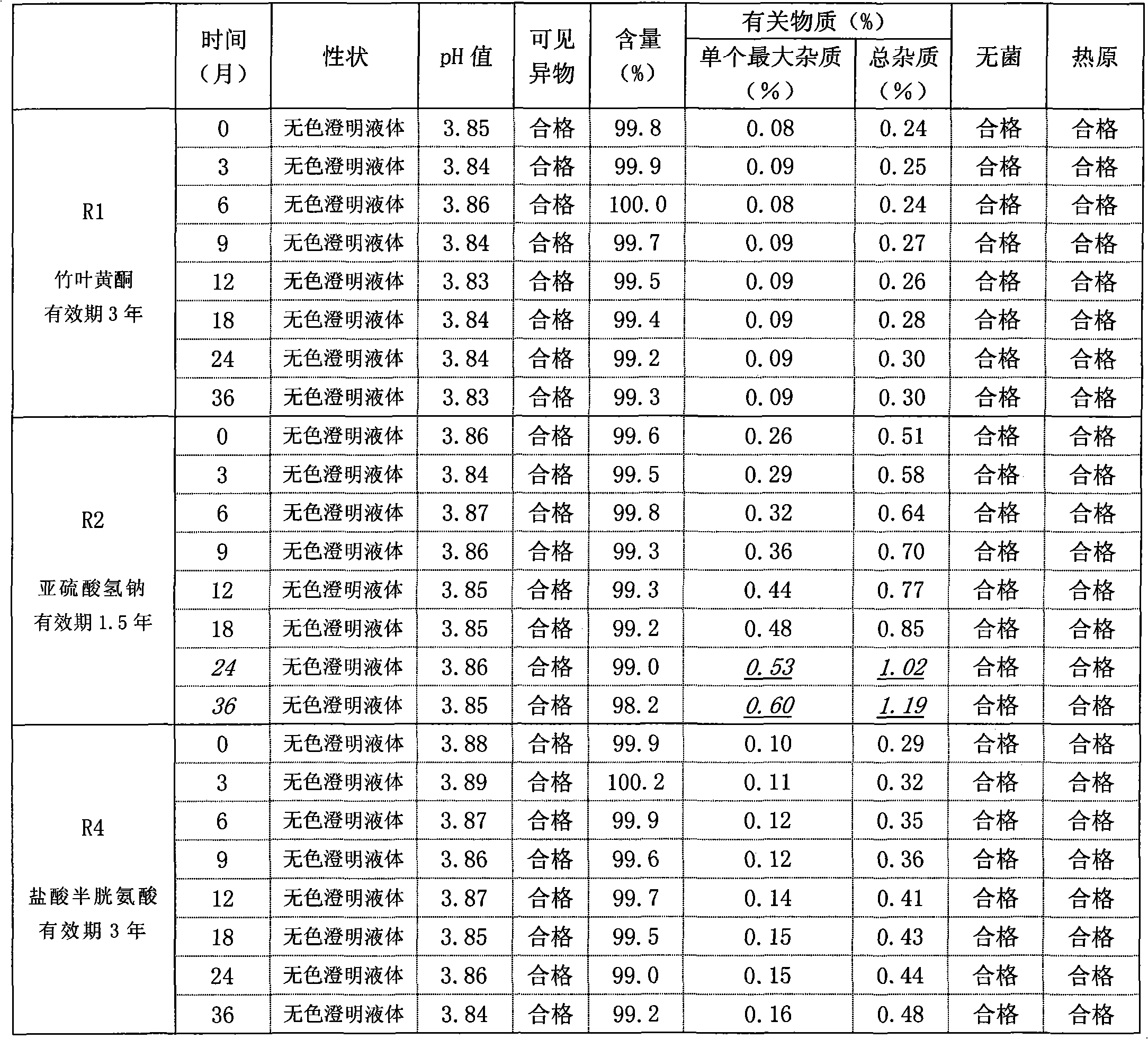
[0057] The sample was inspected after 3 years, and the inspections...
Embodiment 2
[0059] Formula (specification: 5ml: 10mg)
[0060] Raw materials
Dosage
effect
Specification
Edaravone
10g
Main drug
Medicinal
400g
Co-solvent
Medicinal
Bamboo leaf flavonoids
4g
Medicinal
[0061] Add water for injection to
5000ml
solvent
Injection
Potting nitrogen filling
Residual oxygen 1.6%
prevent oxidation
High purity nitrogen
[0062] Dissolve the edaravone in the prescribed amount in propylene glycol, stir until it is completely dissolved, add an aqueous solution of vitamin C and stir, add activated carbon for needles, and adjust the pH with 0.1mol / L hydrochloric acid and / or sodium hydroxide solution after carbon removal, Fill it with nitrogen, seal it in an ampoule, and sterilize it by autoclaving at 115°C for 30 minutes.
[0063] The sample was inspected after 3 years, and the inspections of shape, pH, v...
Embodiment 3
[0065] Formula (Specification: 20ml: 30mg)
[0066] Raw materials
Dosage
effect
Specification
Edaravone
30g
Main drug
Medicinal
Hydroxypropyl Beta Cyclodextrin
160g
Co-solvent
Medicinal
Bamboo leaf flavonoids
24g
antioxidant
Medicinal
Add water for injection to
20000ml
solvent
Injection
Potting nitrogen filling
Residual oxygen 1.5%
prevent oxidation
High purity nitrogen
[0067] Add the prescribed amount of Edaravone, hydroxypropyl beta cyclodextrin, and bamboo leaf flavonoids into water for injection, stir until completely dissolved, add activated carbon for needles, and oxidize with 0.1mol / L hydrochloric acid and / or hydrogen after removing the carbon Adjust the pH with sodium solution, fill it with nitrogen, seal it in an ampoule, and sterilize it by autoclaving at 115°C for 30 minutes.
[0068] The sample was inspected after 3 years, and the inspect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com