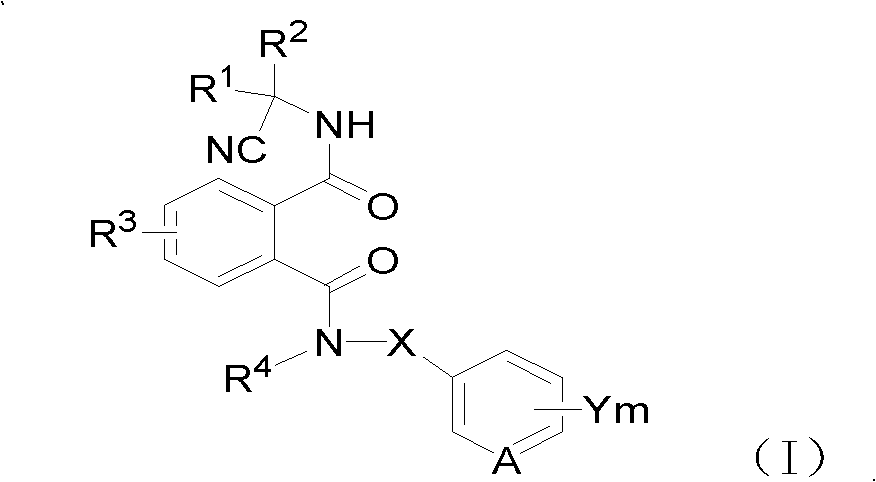
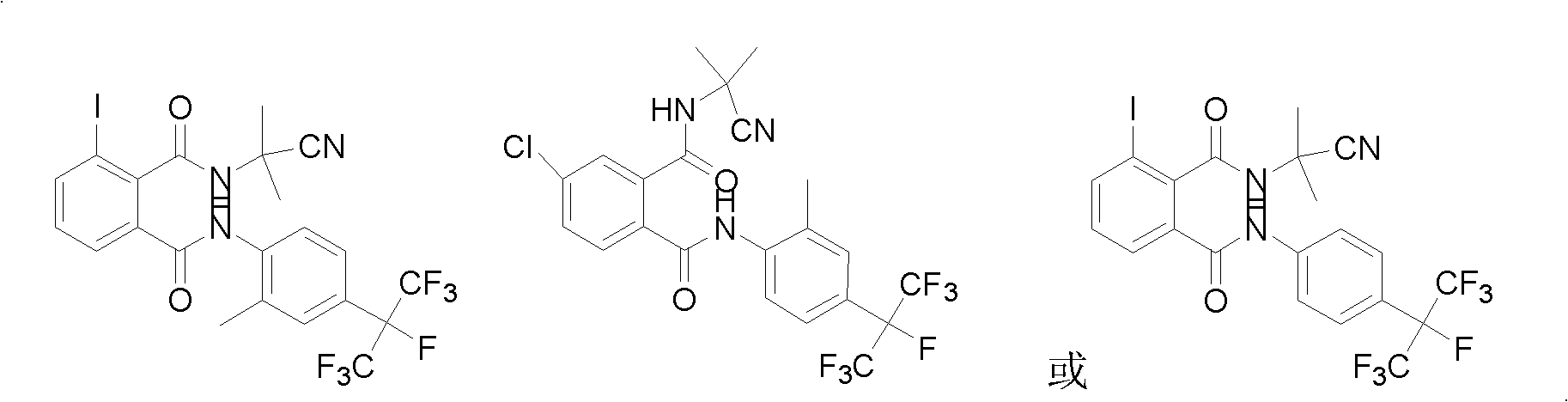
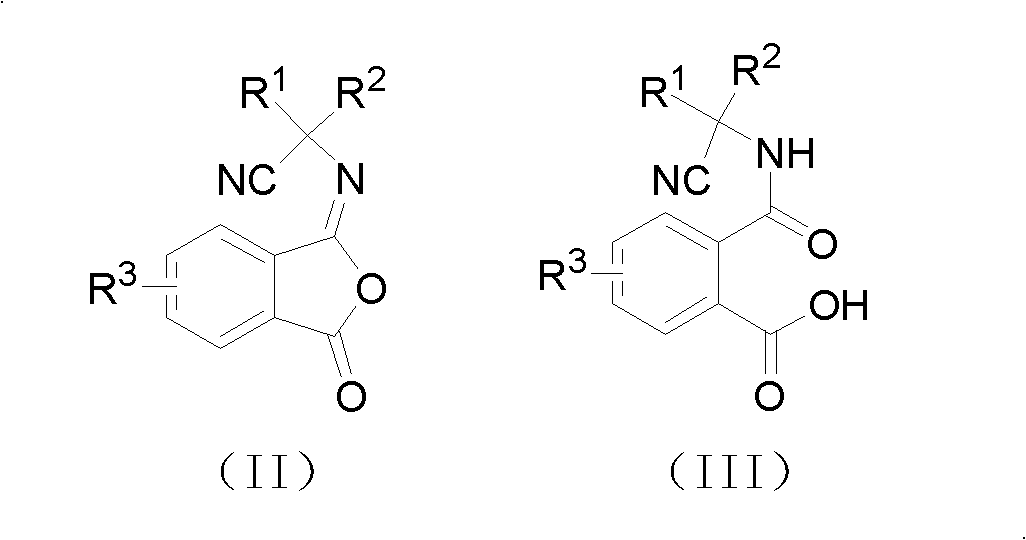
Cyano phthalic diamide compounds, preparation method thereof and use thereof as agricultural chemical pesticide
A technology of phthalamide and compound, applied in the field of phthalamide compounds, can solve the problem that the insecticidal activity of phthalamide compounds is not disclosed and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] 3-iodo-N 1 -(2-Methyl-4-heptafluoroisopropylphenyl)-N 2 - Preparation of (1-methyl-1-cyanoethyl)phthalamide (compound 24)
[0107] Step 1: Synthesis of 3-iodo-N-(1-methyl-1-cyanoethyl)o-amidobenzoic acid
[0108] A mixture of 2-amino-2-dimethylpropionitrile (0.84g, 10mmol) and triethylamine (0.20g, 2mmol) was dissolved in N,N-dimethylacetamide (DMA, 3mL) at room temperature Add slowly to a solution of 3-iodophthalic anhydride (2.74 g, 10 mmol) in DMA (8 mL). The reaction mixture was stirred for 1 h, poured into water, and acidified with dilute hydrochloric acid. The aqueous phase was extracted with ethyl acetate and dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure to give the product as an orange oil, which precipitated out as a solid within a few hours and was washed with a mixture of diethyl ether and n-hexane. Obtained 1.80 g (50%) of a white solid product with a melting point of 141-143° C. and a NMR data of: 1 HNMR (400MH...
Embodiment 2
[0114] 4-Chloro-N 1 -(2,4-Dichlorophenyl)-N 2 Preparation of -(1-methyl-1-cyanoethyl)phthalamide
[0115] (Compound 48)
[0116] Step 1: Synthesis of 4-chloro-N-(1-methyl-1-cyanoethyl)o-amidobenzoic acid
[0117] A mixture of 2-amino-2-dimethylpropionitrile (0.84g, 10mmol) and triethylamine (0.20g, 2mmol) was dissolved in N,N-dimethylacetamide (DMA, 3mL) at room temperature Add slowly to a solution of 4-chlorophthalic anhydride (2.74 g, 10 mmol) in DMA (8 mL). The reaction mixture was stirred for 1 h, poured into water, and acidified with dilute hydrochloric acid. The aqueous phase was extracted with ethyl acetate and dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure to give the product as a clear oil, which precipitated out as a solid within a few hours and was washed with a mixture of diethyl ether and n-hexane. 1.73 g (65%) of the product was obtained, with a melting point of 127-130°C.
[0118] Step 2: Synthesis of 4-chloro-N-(1-...
Embodiment 3
[0124] Embodiment 3 wettable powder formula
[0125]15% of compound (24) (Table 1), 5% of lignosulfonate (M q ), 1% lauryl polyoxyethylene ether (JFC), 40% diatomaceous earth and 44% light calcium carbonate are evenly mixed and pulverized to obtain a wettable powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com