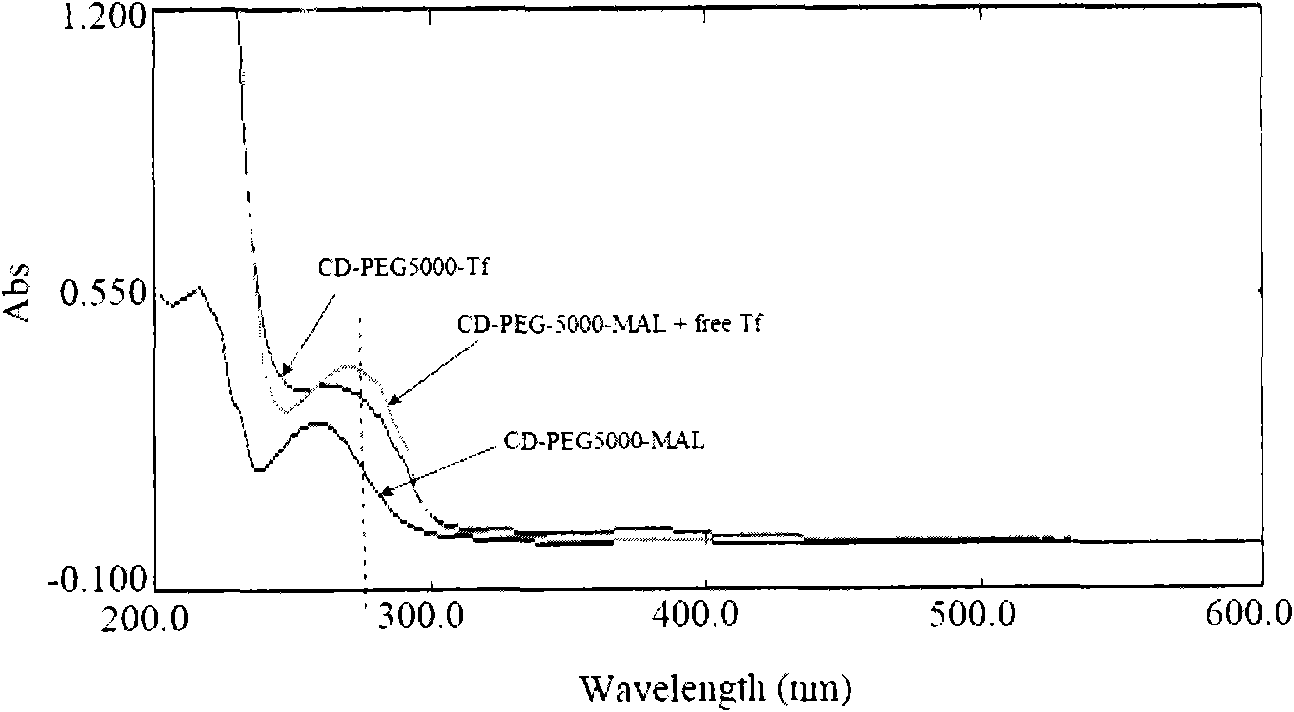
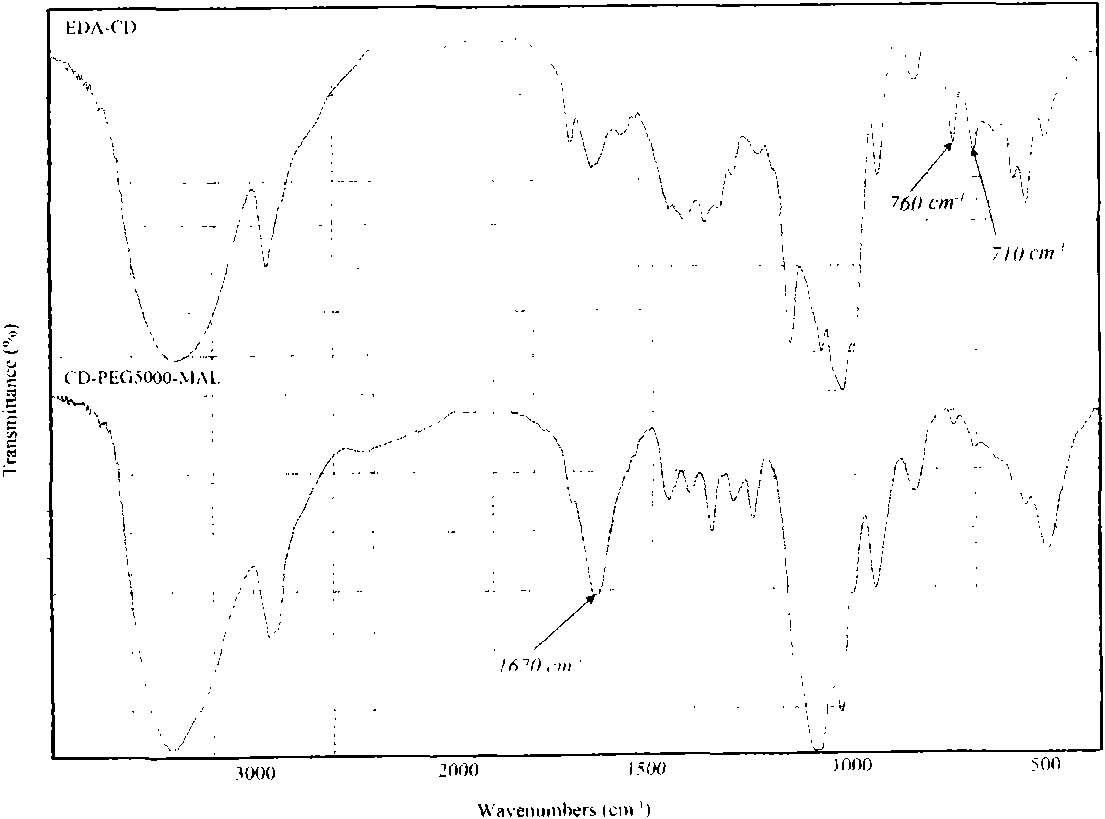
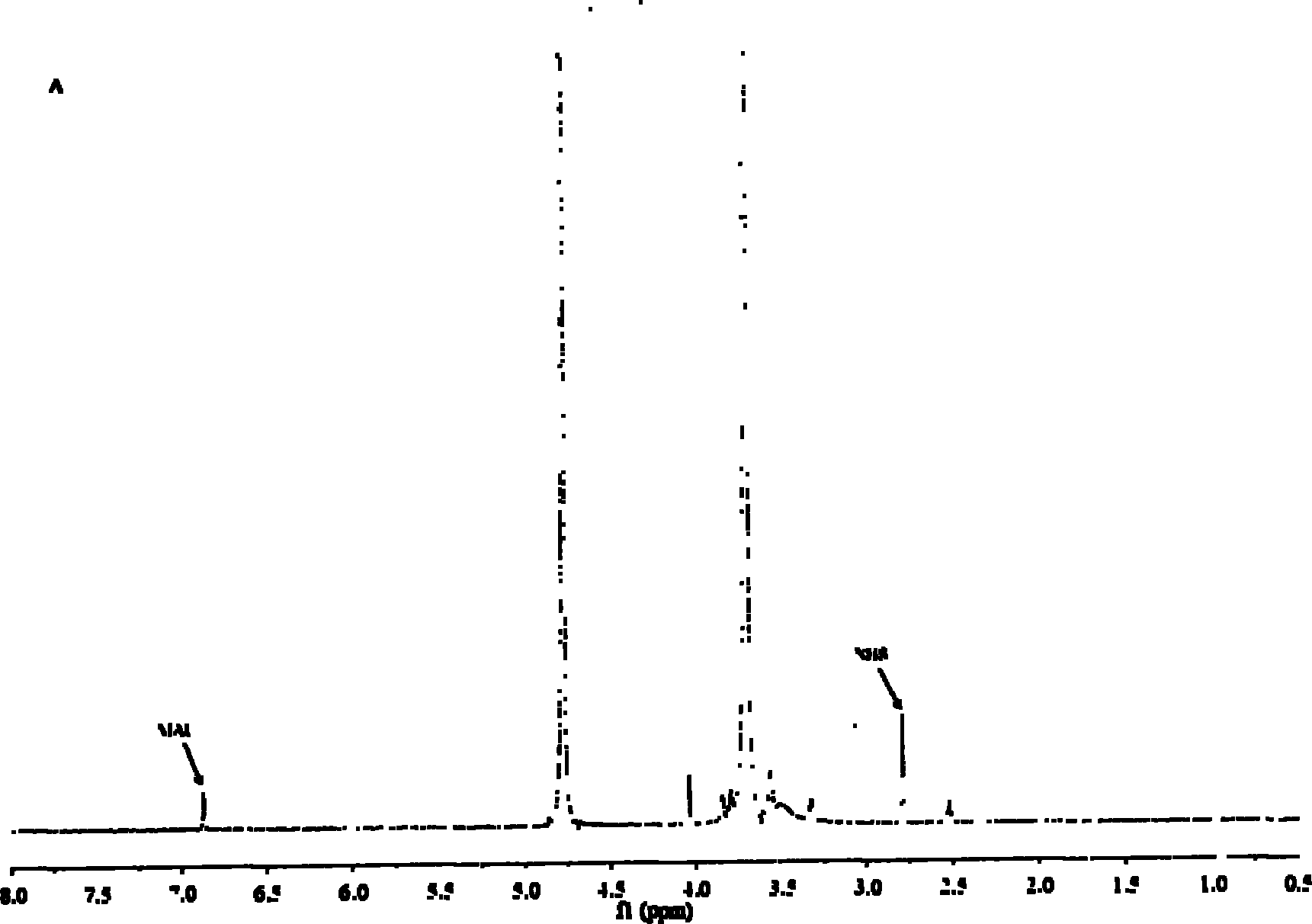
Method for synthesizing brain targeting head modification cyclodextrin (CD) derivative
A synthesis method and cyclodextrin technology, applied in the field of biomedicine, can solve problems such as brain-targeted molecular modification that has not yet been seen, and achieve the effect of improving brain transfer rate, simple method, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]Disperse 60g of β-cyclodextrin evenly in water, add 20ml of 8.25mol / L sodium hydroxide solution at 20°C, drop it in 6 minutes, and the solution becomes clear during the process. After the reaction continued for 1 hour, the reaction temperature was lowered to 2° C., and 15 g of p-toluenesulfonyl chloride acetonitrile solution was added, resulting in a white precipitate. The reaction solution was stirred at room temperature for 2 h, filtered, washed, and the filtrate was adjusted to pH 8 with 10% HCl, and a white solid was precipitated, which was left overnight at 4°C. Filtrate, collect two precipitates, recrystallize three times, and vacuum-dry at 50°C for 6 hours to obtain 6-p-toluenesulfonyl chloride-β-cyclodextrin with a yield of 10%.
[0032] Dissolve 6.5g of 6-p-toluenesulfonyl chloride-β-cyclodextrin in 30ml of anhydrous ethylenediamine, mix well, and react for 4 hours at 80°C under nitrogen protection. After the reaction is completed, cool to room temperature, and ...
Embodiment 2
[0037] Disperse 60g of β-cyclodextrin evenly in water, add 20ml of 8.25mol / L sodium hydroxide solution at 20°C, drop it in 6 minutes, and the solution becomes clear during the process. After the reaction lasted for 1 hour, the reaction temperature was lowered to 2° C., and 20 g of p-toluenesulfonyl chloride acetonitrile solution was added to produce a white precipitate. The reaction solution was stirred at room temperature for 2 h, filtered, washed, and the filtrate was adjusted to pH 8 with 10% HCl, and a white solid was precipitated, which was left overnight at 4°C. Filtrate, collect two precipitates, recrystallize three times, and vacuum-dry at 50°C for 6 hours to obtain 6-p-toluenesulfonyl chloride-β-cyclodextrin with a yield of 7.8%, and a small amount of 2-p-toluenesulfonyl chloride-β- Cyclodextrin by-products.
[0038] Dissolve 6.5g of 6-p-toluenesulfonyl chloride-β-cyclodextrin in 30ml of anhydrous ethylenediamine, mix well, and react for 4 hours at 80°C under nitroge...
Embodiment 3
[0043] Disperse 60g of β-cyclodextrin evenly in water, add 20ml of 8.25mol / L sodium hydroxide solution at 20°C, drop it in 6 minutes, and the solution becomes clear during the process. After the reaction continued for 1 hour, the reaction temperature was lowered to 2° C., and 15 g of p-toluenesulfonyl chloride acetonitrile solution was added, resulting in a white precipitate. The reaction solution was stirred at room temperature for 2 h, filtered, washed, and the filtrate was adjusted to pH 8 with 10% HCl, and a white solid was precipitated, which was left overnight at 4°C. Filtrate, collect two precipitates, recrystallize three times, and vacuum-dry at 50°C for 6 hours to obtain 6-p-toluenesulfonyl chloride-β-cyclodextrin with a yield of 10%.
[0044] Dissolve 6.5g of 6-p-toluenesulfonyl chloride-β-cyclodextrin in 30ml of anhydrous ethylenediamine, mix well, and react for 4 hours at 80°C under nitrogen protection. After the reaction is completed, cool to room temperature, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com