Method for preparing solar grade silicon from silica serving as raw material
A solar-grade, silica technology, applied in chemical instruments and methods, silicon compounds, non-metallic elements, etc., can solve the problems of large environmental pollution and high production cost, and achieve the effects of low environmental pollution, low production cost and low power consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
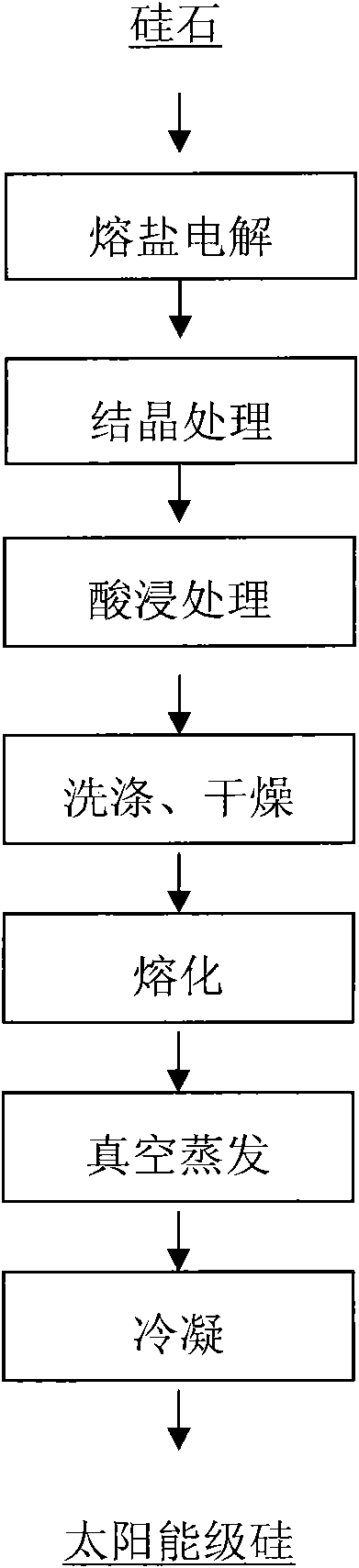
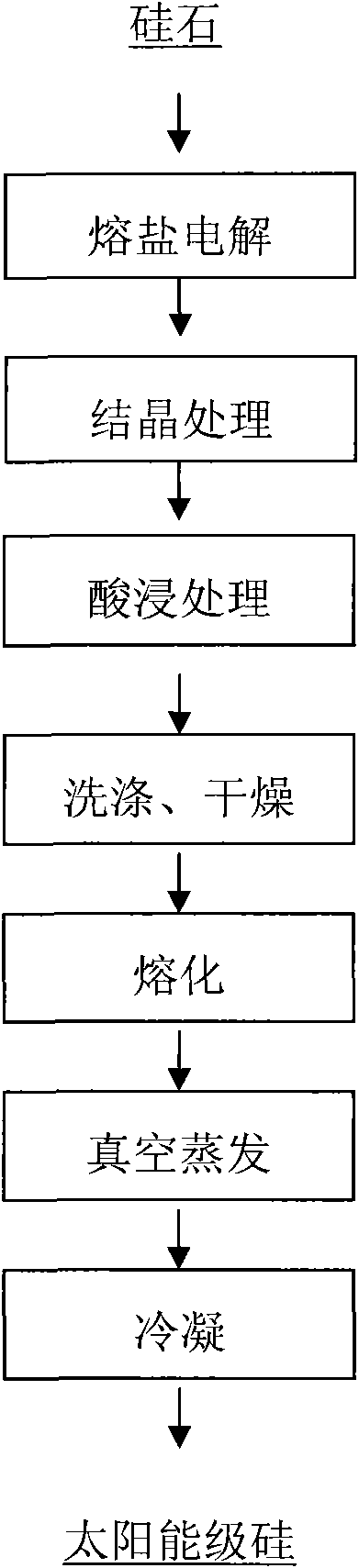
[0022] A. Using silica as raw material and CaCl 2 It is a molten salt electrolyte, and the conventional reduction is carried out through the molten salt electrolyte at 900 ° C, so that the elemental silicon in the silica is directly electrolytically reduced to elemental silicon;
[0023] B. Add CuS to the elemental silicon in step A at a mass ratio of 1:3, and pass argon gas into it at 1000° C. for conventional crystallization treatment to obtain crystalline silicon;
[0024] C. according to the solid-liquid mass ratio of 1:5, the crystalline silicon of step B is placed in the hydrochloric acid solution that concentration is 5mol / L, pickling 20 hours;
[0025] D. Take out the acid-leached silicon, wash it with water 5 times, and dry it at 150°C for 2 hours;
[0026] E. Melting the dried silicon at 1500°C;
[0027] F. Put the molten silicon at a temperature of 1650°C and a pressure of 1×10 -2 Carry out vacuum evaporation under the vacuum condition of Pa;
[0028] G. After c...
Embodiment 2
[0030] A. Using silica as raw material and CaCl 2 It is a molten salt electrolyte, which is conventionally reduced by a molten salt electrolyte at 600°C, so that the elemental silicon in the silica is directly electrolytically reduced to elemental silicon;
[0031] B. Add CrCl to the elemental silicon obtained in step A at a mass ratio of 1:9 3 , and pass argon at 600°C for conventional crystallization treatment to obtain crystalline silicon;
[0032] C. according to the solid-liquid mass ratio of 1:1, the crystalline silicon of step B is placed in the hydrochloric acid solution that concentration is 3mol / L, acid leaching for 48 hours;
[0033] D. Take out the acid-leached silicon, wash it with water 4 times, and dry it at 120°C for 3 hours;
[0034] E. Melting the dried silicon at 1650°C;
[0035] F. Vacuum evaporation of the melted silicon under vacuum conditions with a temperature of 1600° C. and a pressure of 10 Pa;
[0036] G. After condensing the molten silicon after...
Embodiment 3
[0038] A. Using silica as raw material and CaCl 2 It is a molten salt electrolyte, which is conventionally reduced by a molten salt electrolyte at 1000°C, so that the elemental silicon in the silica is directly electrolytically reduced to elemental silicon;
[0039] B. Add FeCl to the elemental silicon obtained in step A at a mass ratio of 1:6 3 and VF 3 , and pass argon gas at 1200°C for conventional crystallization treatment to obtain crystalline silicon;
[0040] C. According to the solid-liquid mass ratio of 1:8, the crystalline silicon of step B is placed in the hydrochloric acid solution that concentration is 1mol / L, pickling 2 hours;
[0041] D. Take out the acid-leached silicon, wash it twice with water, and dry it at 40°C for 2.5 hours;
[0042] E. Melting the dried silicon at 1450°C;
[0043] F. Carry out vacuum evaporation of the melted silicon under vacuum conditions with a temperature of 1620°C and a pressure of 5Pa;
[0044] G. Condensing the molten silicon ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com