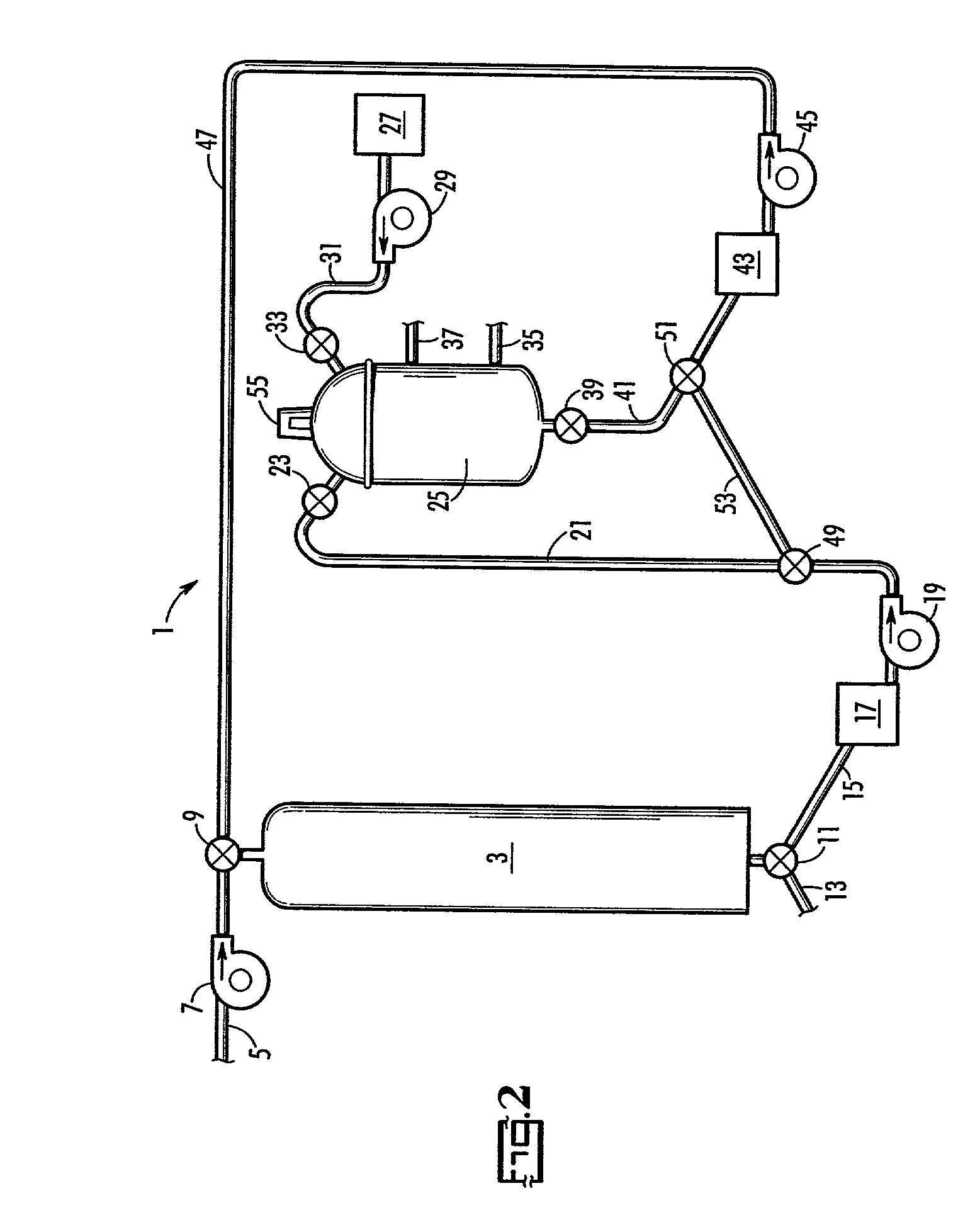
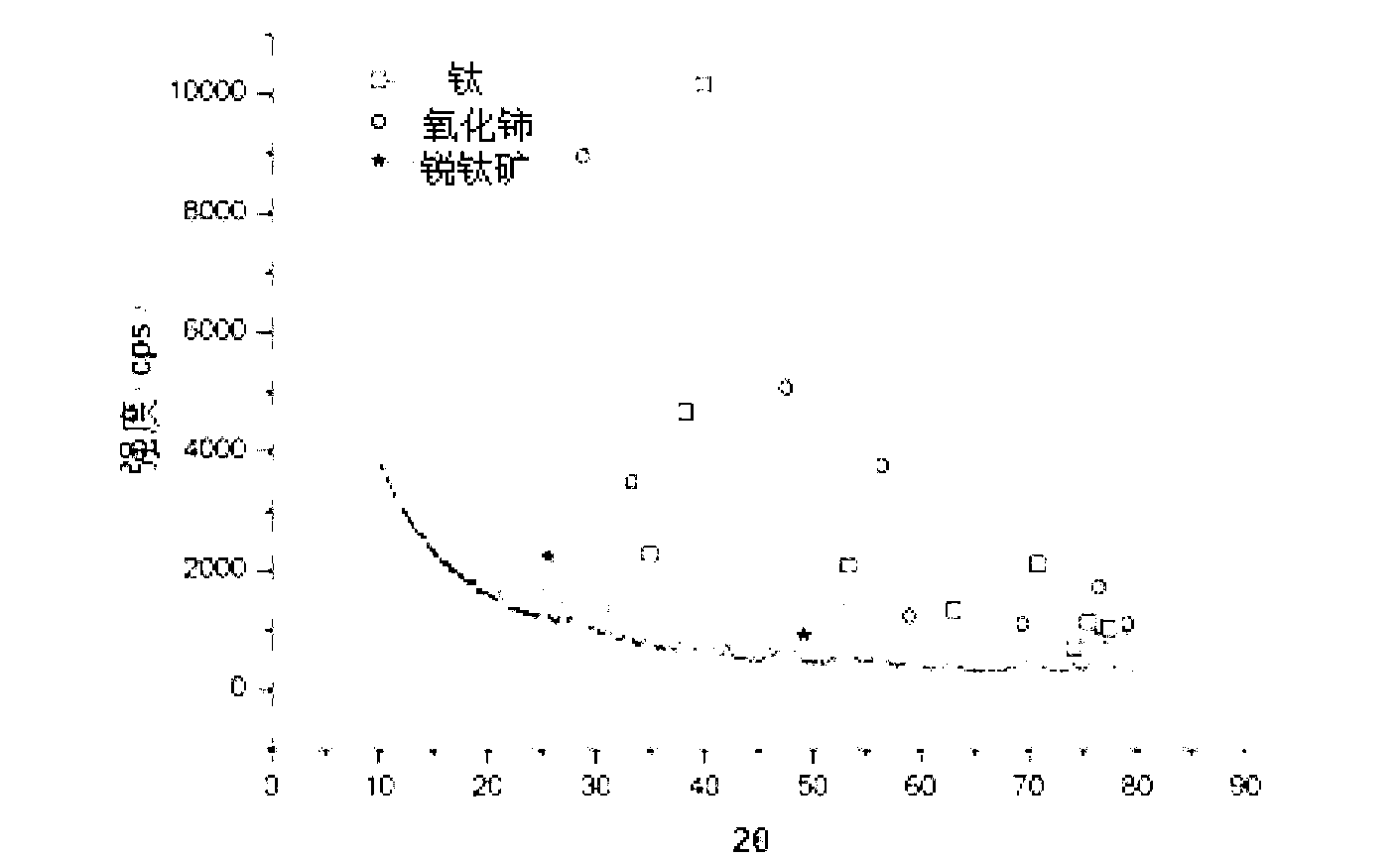
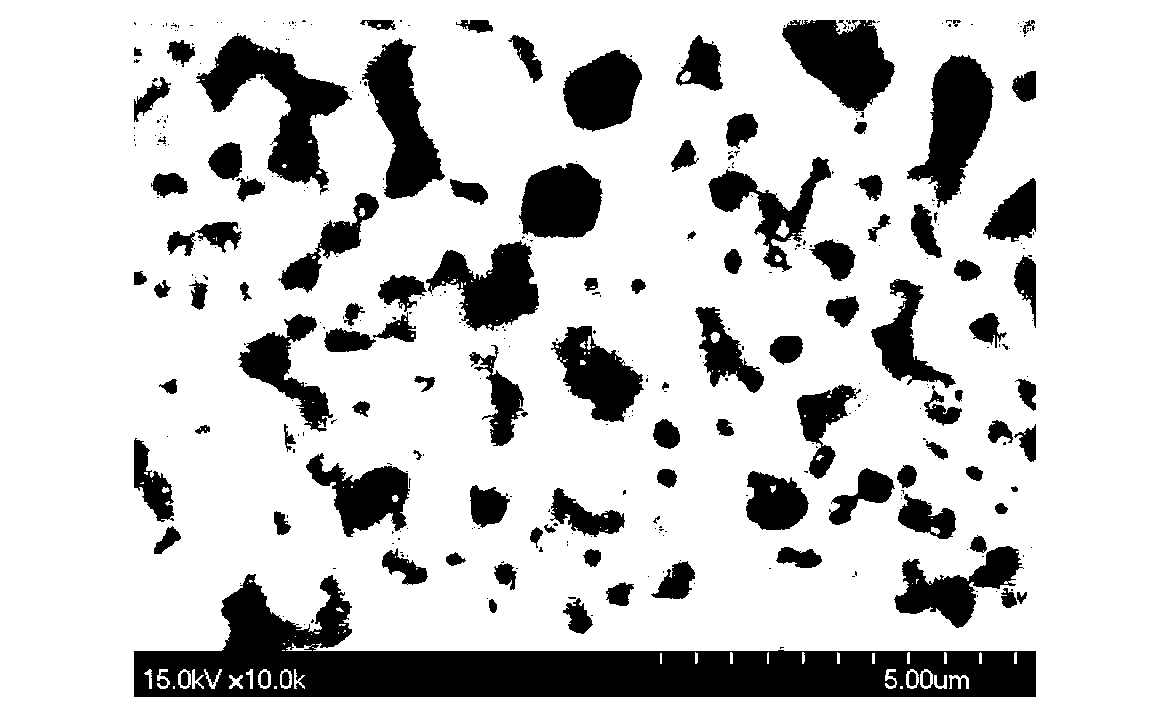
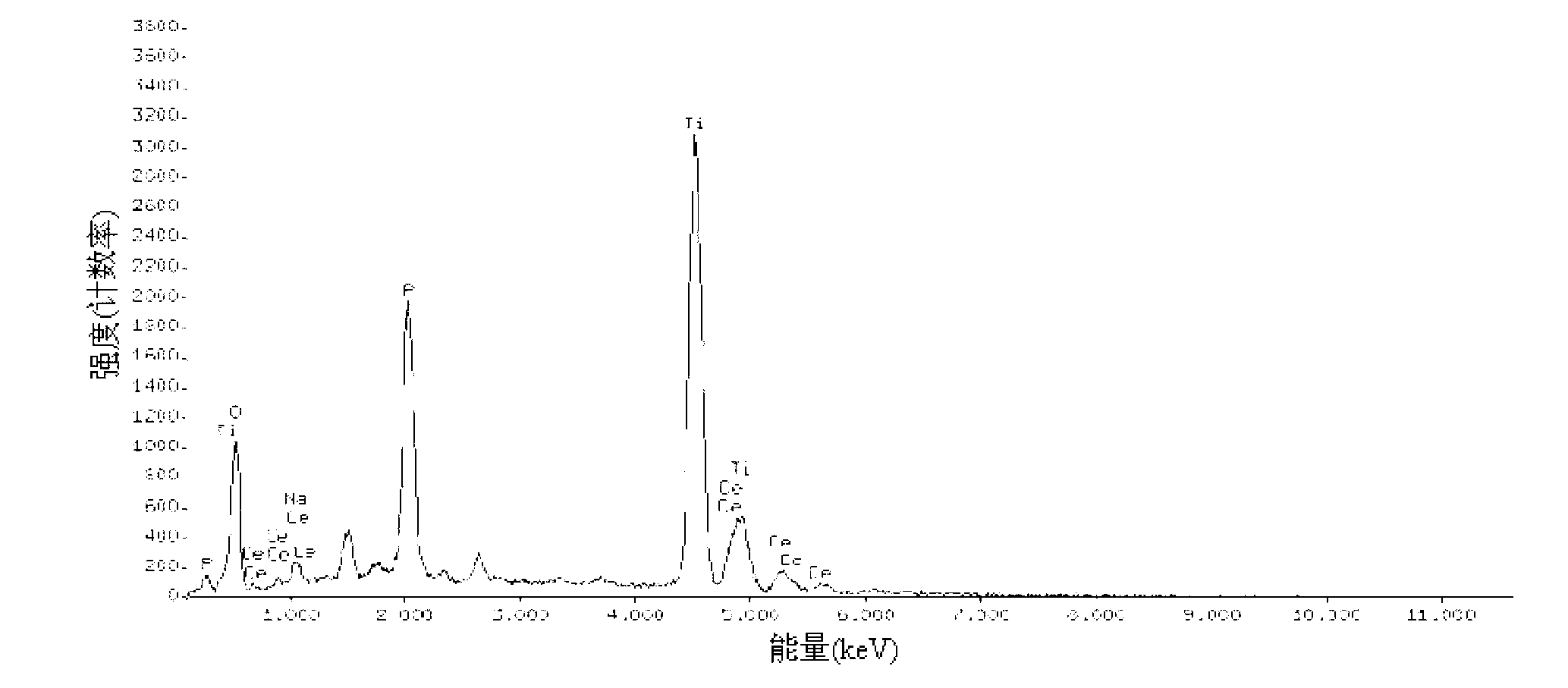
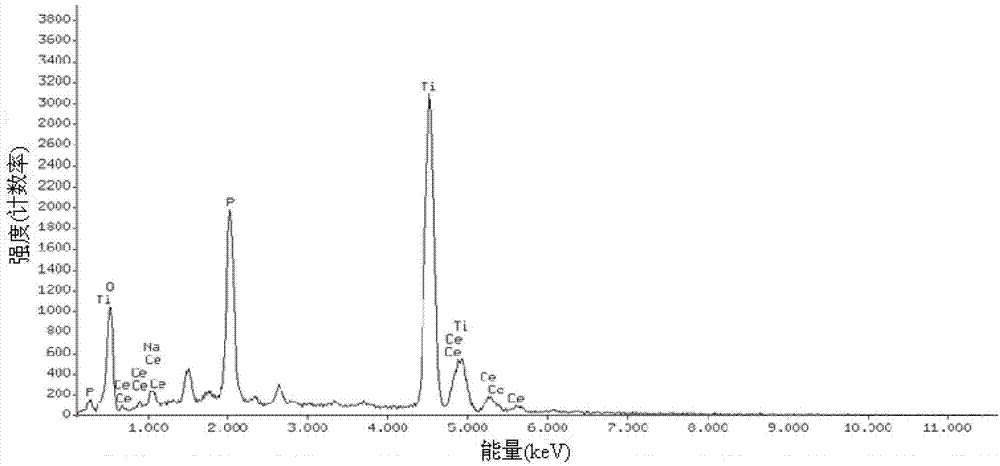
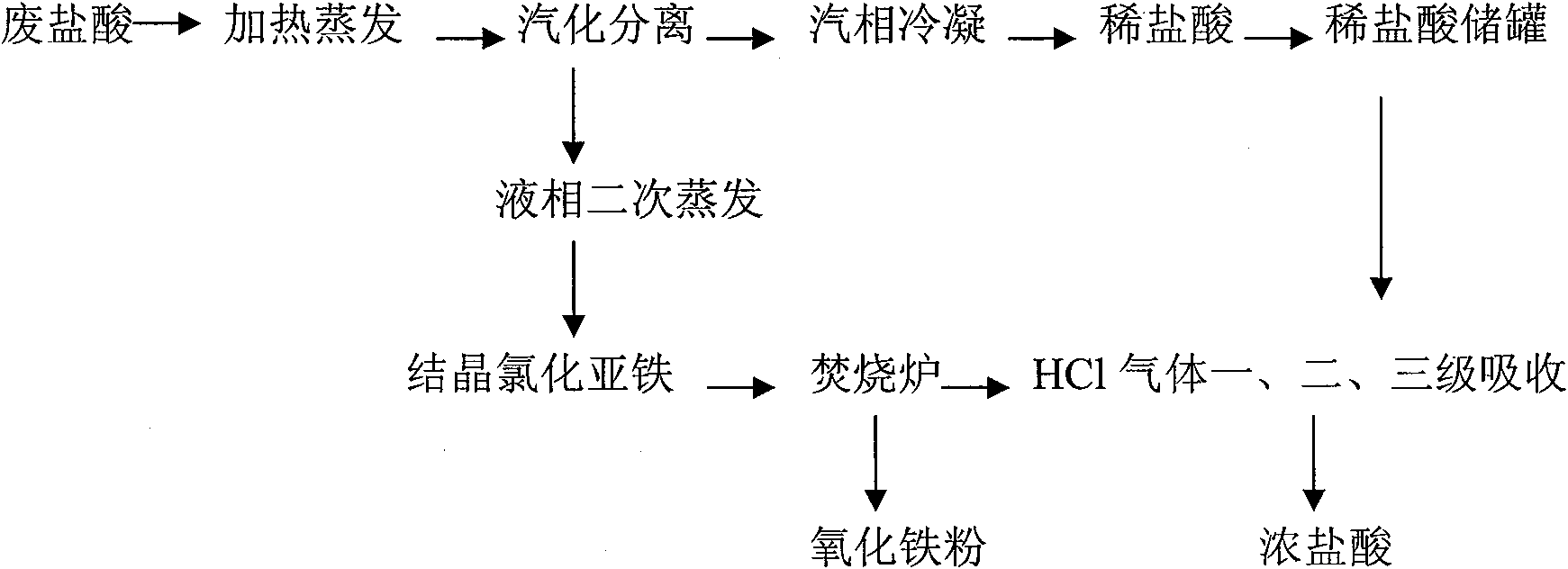
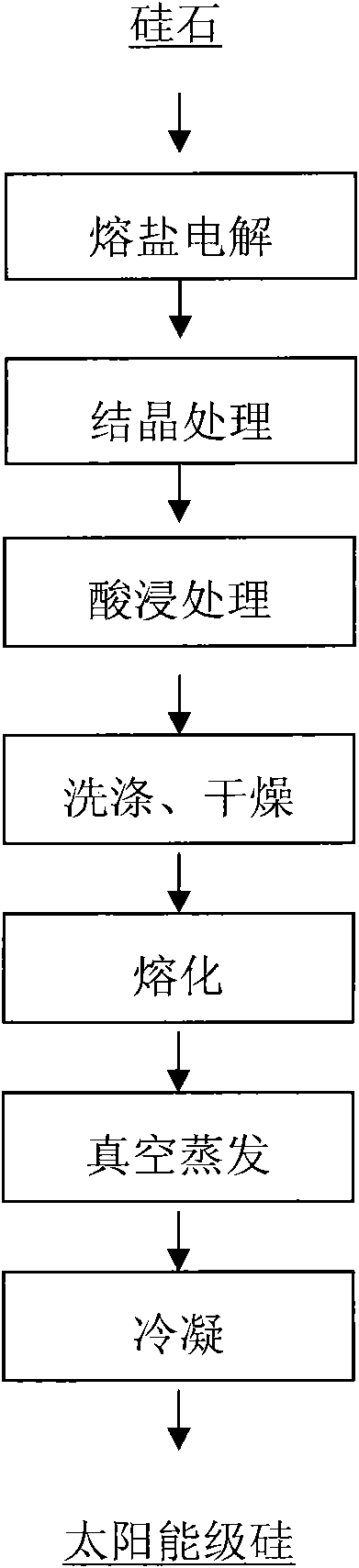
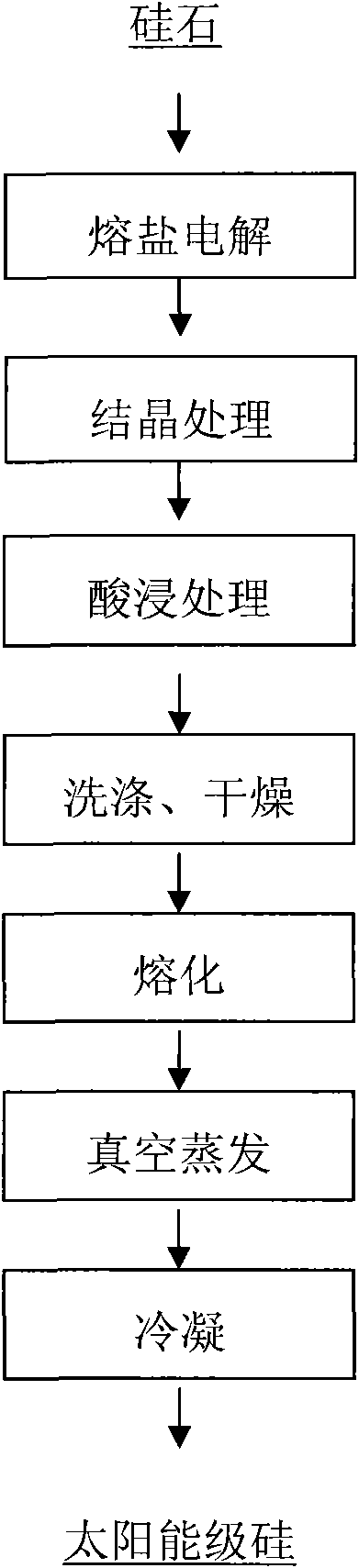
Patents
Literature
176 results about "Hydrochloric Acid Liquid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The chemical compound hydrochloric acid is the aqueous (water-based) solution of hydrogen chloride gas (HCl). It is a strong acid, the major component of gastric acid and of wide industrial use. Hydrochloric acid must be handled with appropriate safety precautions because it is a highly corrosive liquid.
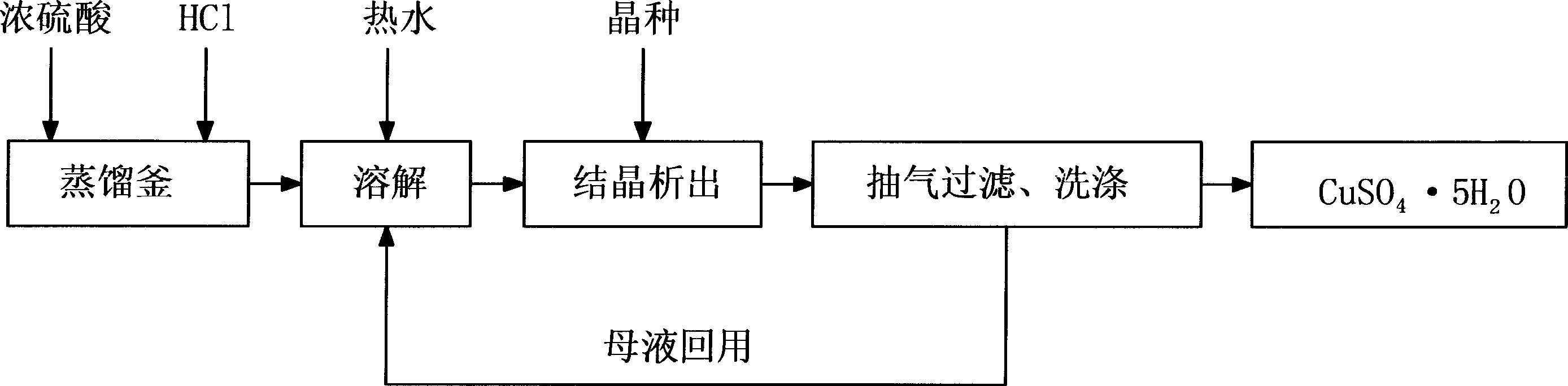
Method for recovering hydrochloric acid and copper sulfate from acidic etching liquid
This invention is a method of recovering chlorhydric acid and copper sulfate from acid etching solution. The procedures are that (1) recovering chlorhydric acid. The acid etching waste solution is filled in acid resistant distillation plant, then concentrated sulfuric acid is added, then they are heated to boiling state and the chlorhydric acid is recovered through distillation, the concentration of the concentrated sulfuric acid is 98% or more than 98%. The adding on is more than 5-15% than that based on the theoretical value of copper ion fully reacted with sulfuric acid in the distillation. (2) Recovering copper sulfate. First, the residue in chlorhydric acid recovery is watering dissolved to thoroughly decompose the copper sulfate in the residue; the adding quantity of the water is 30-70% volume of acid etching waste solution. Secondly, copper sulfate is seed out through the changing of high temperature to low temperature; the temperature change is controlled between 40 to 60 degrees centigrade. Thirdly, copper sulfate is got after the copper sulfate crystalline is filtrated and washed. The simplest method is used to recover the useful resource in etching waste liquor in this invention. The technique equipment is simple, operation is convenient, and there is no environment pollution, so it has wide using value.
Owner:苏州天地环境科技有限公司
Method for removing copper, manganese, iron, calcium and magnesium from cobalt salt solution through three-section extraction method
The invention belongs to the technical field of appliances and particularly relates to a method for removing copper, manganese, iron, calcium and magnesium from a cobalt salt solution through a three-section extraction method. The method for removing copper, manganese, iron, calcium and magnesium from the cobalt salt solution through the three-section extraction method is characterized in that during first-section extraction, p204 extract liquid is used for extracting calcium, copper, manganese, zinc and iron out; and 0.6 N-1.0 N of hydrochloric acid liquid and 0.6 N-1.0 N of nitric acid liquid are selected, the washing level is 4 to 6, the water phase flow is stopped till outlet 1-2 levels of water phases are kept free of the obvious red color, the PH of a washing water outlet is 3.0 to 4.0, and anti-iron liquid is 6N HCl. The method for removing copper, manganese, iron, calcium and magnesium from the cobalt salt solution through the three-section extraction method has the beneficial effects that the technology is simple and reliable, the equipment investment is saved, the impurity separating and removing effect is good, the production efficiency is high, and the environment cannot be polluted.
Owner:德清县立荣金属粉末有限公司
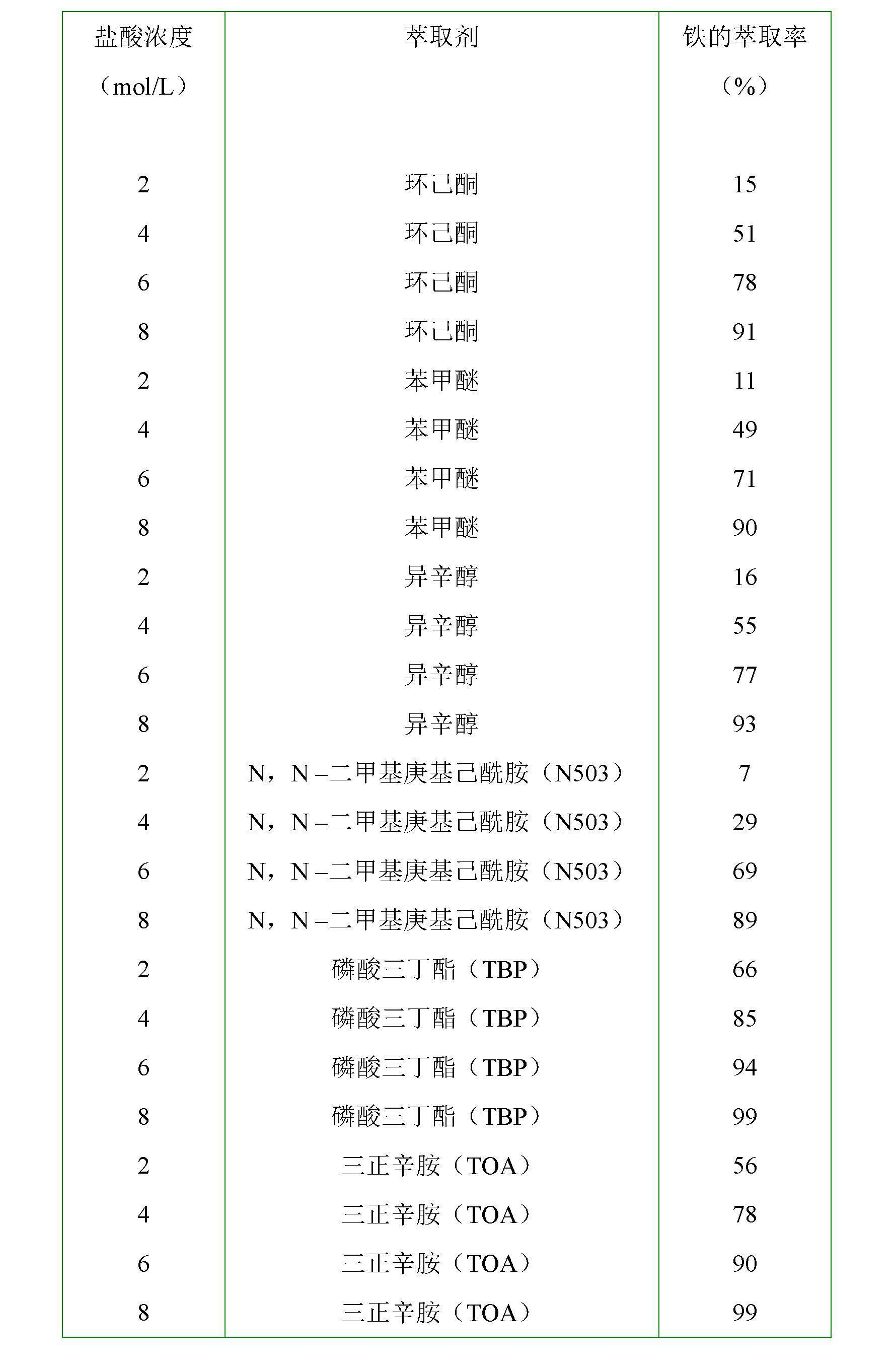
Method for selectively separating iron from hydrochloric acid solution containing ferrous chloride
InactiveCN102660678AReduce concentrationThe driving force of the oxidation reaction is largeProcess efficiency improvementReaction temperatureHydrochloric Acid Liquid
The invention discloses a method for selectively separating iron from hydrochloric acid solution containing ferrous chloride. The method comprises the following steps of: with hydrochloric acid solution containing ferrous chloride as a water phase, adding the water phase and an organic phase into extraction equipment to form a mixed solution; introducing gas containing oxygen into the mixed solution; simultaneously oxidizing and extracting by stirring, wherein the reaction temperature is 25-40DEG C and the reaction time of each stage of extraction is at least 15 minutes; after the reaction time reaches, stopping stirring and introducing the gas containing the oxygen; standing a reaction solution for at least 3 minutes; and then separating an extraction solution and the organic phase, wherein the mixture ratio of the organic phase to the water phase is in the limit of the mol ratio of an extraction agent in the organic phase to an iron ion in the water phase being (2-5) to 1 and the total introduction quantity of the gas containing the oxygen is 2-10 times the theoretical quantity of the gas containing the oxygen required by complete oxidation of ferrous chloride in the mixed solution into ferric trichloride.
Owner:SICHUAN UNIV
Preparation method of nanoscale stannic oxide hollow sphere
InactiveCN102583266ANo pollution in the processRaw materials are cheap and easy to getNanotechnologyElemental selenium/telluriumGas detectorPotassium hypochlorite
The invention belongs to the field of nano materials and relates to a preparation method of a nanoscale stannic oxide hollow sphere. The method is characterized in that stannous salts and oxidants serve as reaction materials and a hydro-thermal method is used for preparation under the acidic condition at the PH value between 1.0 and 4.0; the stannous salts consist of at least one of stannous sulfate, stannous chloride and stannous oxalate; the oxidants consist of at least one of hydrogen peroxide, chloric acid, hypochlorous acid, potassium chlorate, sodium chlorate, potassium hypochlorite and sodium hypochlorite; and PH value of solutions is adjusted by one of sulphuric acid, hydrochloric acid, oxalic acid and sodium hydroxide. The preparation method does not use any sacrificial templates and organic solvents and is environmentally friendly, simple in process, mild in reaction condition, low in energy consumption, easy to control, high in product purity, and suitable for large-scale industrial production. The nanoscale stannic oxide hollow sphere can be applicable to gas sensors, lithium ion batteries, catalysts and photoelectronic devices.
Owner:SHANDONG UNIV
Acid-washing waste acid reproduction method
InactiveCN101041902AImprove qualityTo achieve the purpose of recyclingAcid washingHydrochloric Acid Liquid
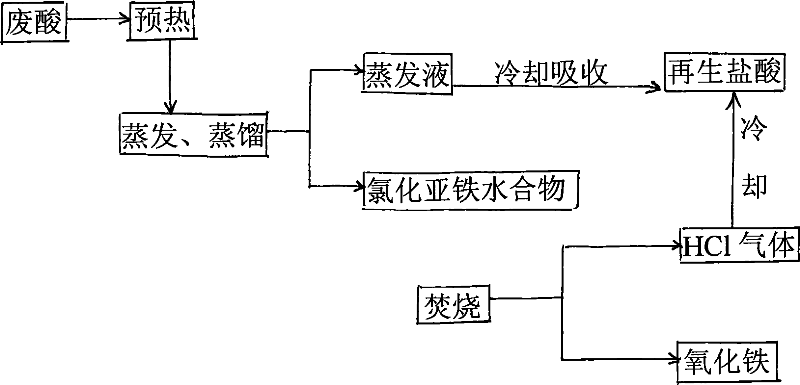
The invention discloses a regenerating method of waste pickling acid, which comprises the following steps: evaporating alcaine from waste alcaine liquid of rust pickling steel product; cooling; recycling; sintering iron chloride; cooling the sintered hydrogen chloride gas to form alcaine; recycling; collecting the sintered ferric oxide; circulating alcaine effectively.
Owner:黄健
Decalcified dental matrix of mammal and preparation method thereof
InactiveCN104147644AGood induction of bone regenerationConvenient sourceSurgeryProsthesisMammalDisinfectant
The invention relates to a decalcified dental matrix of a mammal and a preparation method thereof. The raw material is from permanent teeth of the mammal. The preparation method comprises the following steps: (1) collecting permanent teeth of the mammal for later use; (2) soaking the permanent teeth in a 50% 84 disinfectant for sterilizing, wherein the time for sterilizing is 45 minutes and the temperature is 35-40DEG C; (3) removing useless parts of the teeth; (4) smashing tooth tissues into particles; (5) sterilizing the smashed tooth tissue particles, wherein the time for sterilizing is 45 minutes and the temperature is 35-40DEG C; (6) putting the treated tooth tissue particles into 0.65-0.72 equivalent weight of hydrochloric acid liquid for soaking for 10-16 hours at 34-38DEG C to finish decalcification; and (7) drying at low temperature, soaking in hydrogen peroxide for 30 minutes, so as to obtain the decalcified dental matrix of the mammal. The decalcified dental matrix of the mammal has better inductive bone regenerative action, and does not carry any pathogenic factor after being prepared into final products.
Owner:李成林
Extraction liquid and extraction method for sulphuric acid in sulfuric acid hydrolysate prepared with plant fibre
InactiveCN101279722ALow costImprove economySulfur-trioxide/sulfuric-acidLiquid solutions solvent extractionHigh concentrationHydrolysate
The invention discloses an extract liquid of sulfuric acid from the sulfuric acid hydrolysate of plant fibrous materials and an extraction method thereof. The extract liquid consists of an amine extractant, a diluent and a phase modifier. The sulfuric acid hydrolysate of the plant fibrous materials is fully contacted with the extract liquid to obtain sugar solution containing a little sulfuric acid and the extract liquid containing lots of sulfuric acid; and the extract liquid containing lots of sulfuric acid is fully contacted with stripping agent ammonia or water to obtain the extract liquid containing a little sulfuric acid and ammonium sulfate solution or sulfuric acid solution, the extract liquid and the sulfuric acid solution can be used circularly and ammonium sulfate can be used as fertilizer. The sulfuric acid in the sulfuric acid hydrolysate of the plant fibrous materials can be extracted and recycled simply and economically by the method of the invention, and especially the sulfuric acid is recycled when dilute sulfuric acid with comparatively high concentration is used for hydrolyzing hemicellulose under normal pressure, which has application significance as an acidproof and voltage withstanding cooking boiler is not needed. The extract liquid and the method of the invention are also applicable to extracting and recycling acid when inorganic acid such as hydrochloric acid is used as the hydrolysis catalyst of the plant fibrous materials.
Owner:陈培豪
Method for producing high-purity concentrated hydrochloric acid by adopting hydrogen chloride gas containing chlorosilane
ActiveCN101628710AHigh purityImprove production stabilityChlorine/hydrogen-chloride purificationPhysical chemistryWater circulation
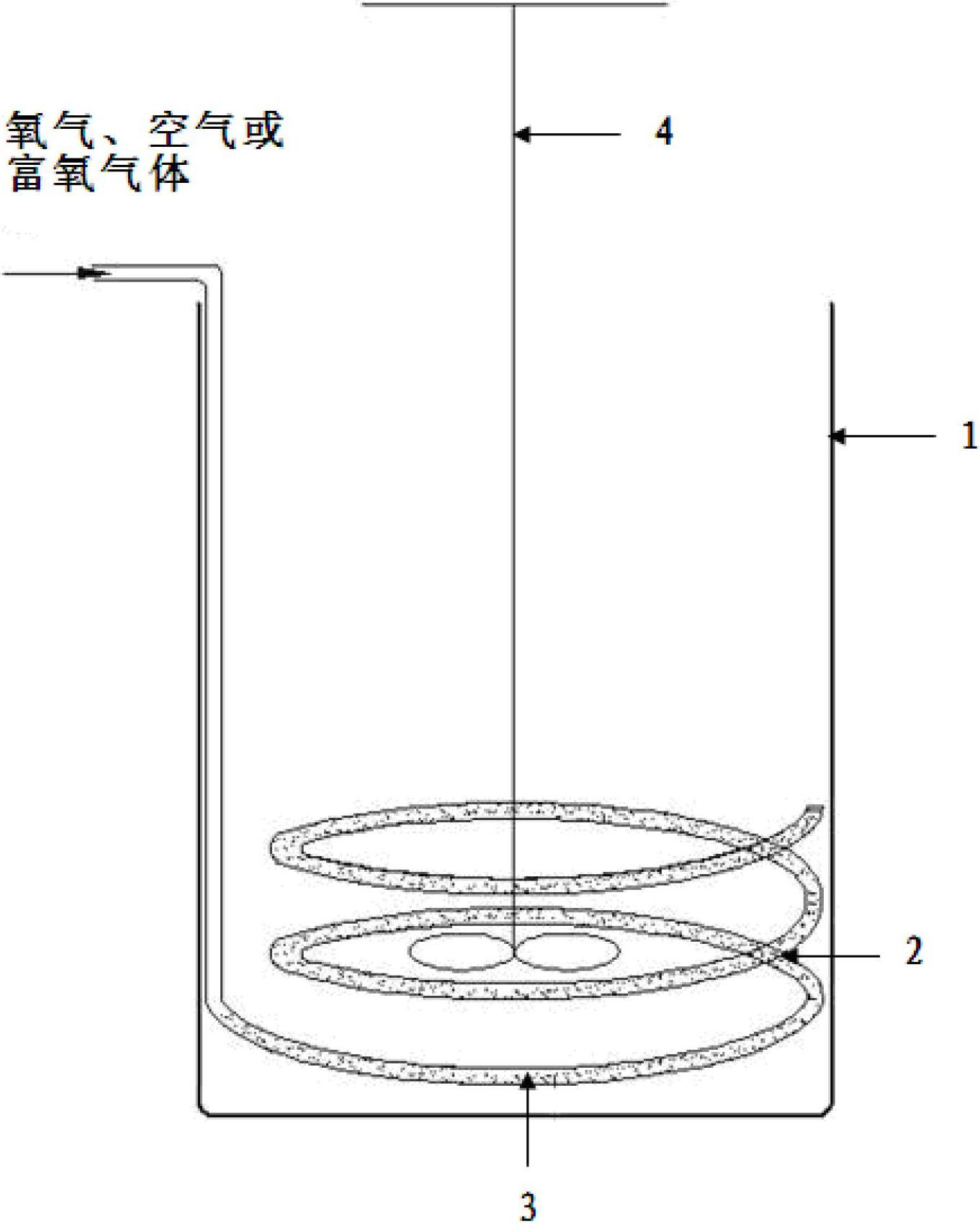
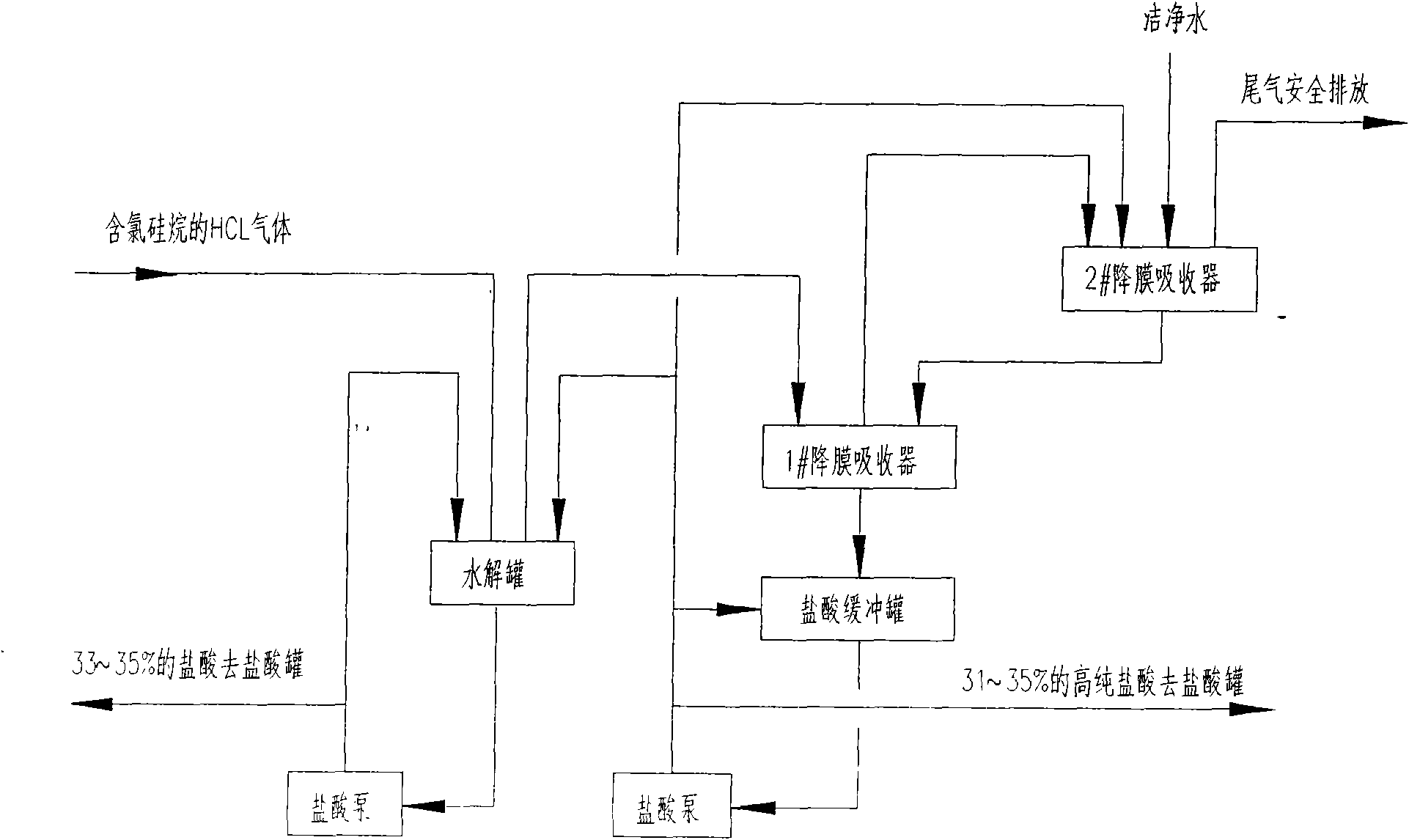
The invention discloses a method for producing high-purity concentrated hydrochloric acid by adopting hydrogen chloride gas containing chlorosilane, which comprises the following steps: injecting hydrogen chloride gas containing chlorosilane into a hydrolyzer, carrying out hydrolyzation reaction with water in concentrated hydrochloric acid in the hydrolyzer, removing chlorosilane in the gas, adopting water circulation to absorb hydrogen chloride in the gas in falling film absorbers after the gas passes through at least two serial falling film absorbers in sequence to obtain concentrate hydrochloric acid, and discharging tail gases after the hydrogen chloride is absorbed. By utilizing the method, the high-purity concentrated hydrochloric acid is produced by adopting hydrogen chloride gas containing chlorosilane, thus the problem that pure hydrochloric acid can not be obtained in the traditional method that water is directly used for absorbing hydrochloric acid is solved; the production stability, the mutual independence and the flexible operability of a polysilicon production device are increased; and meanwhile, the high-purity hydrochloric acid can be also obtained, recycled and sold as a by-product.
Owner:SINOPEC NANJING ENG & CONSTR
Acid pickling process and acid pickling system for active carbon
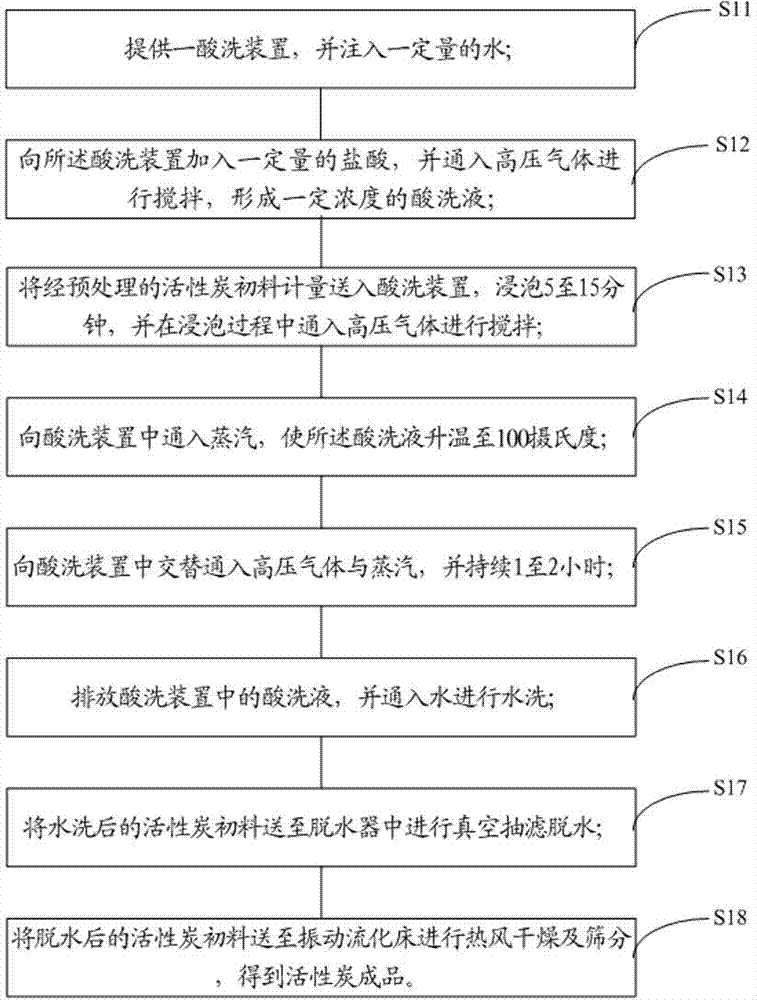
The invention provides an acid pickling process for active carbon. The acid pickling process comprises the following steps of: providing an acid pickling device and injecting a certain amount of water in the device; adding a certain amount of hydrochloric acid to the acid pickling device, and introducing high-pressure gas and stirring to form acid pickling solution with a certain concentration; measuring preprocessed active carbon material and feeding the active carbon material to the acid pickling device, soaking and stirring; introducing steam for heating and then alternately introducing high-pressure gas and steam for 1-2 hours; discharging the acid pickling solution at the end of acid pickling, and washing the active carbon material with water; and dehydrating, drying and screening to obtain the active carbon finished product. The invention also provides an acid pickling system for active carbon. By adopting the acid pickling process and the acid pickling system for active carbon, the semi-automatic continuous work of the whole acid pickling process can be realized, the sealing effect of the whole acid pickling process is realized, and pollution to the environment and corrosion to peripheral equipment can be avoided.
Owner:INST OF URBAN ENVIRONMENT CHINESE ACAD OF SCI
Method for comprehensively treating waste hydrochloric acid and alkaline sludge generated in steel wire rope processing process
InactiveCN104843957AReduce pollutionReduce processing costsSludge treatmentSludgePulp and paper industry
The invention provides a method for comprehensively treating waste hydrochloric acid and alkaline sludge generated in the steel wire rope processing process. The waste hydrochloric acid generated in the steel wire rope processing process is added into the alkaline sludge generated in the steel wire rope processing process; then, the stirring is carried out, so that the sufficient reaction is taken, and a suspension with a small number of acid non-soluble substances is formed; when the PH value in the suspension is not changed, the stirring is stopped; then, the small amount of acid non-soluble substances in the suspension are filtered away, so that acid filter liquid containing various metal ions can be obtained; the PH value of the acid filter liquid is controlled to be in a range being 0 to 1. The mass ratio of the hydrochloric acid to the alkaline sludge is (1-8):1. The hydrochloric acid concentration in the waste hydrochloric acid is not lower than 5 percent, and the hydrochloric acid is added for regulation when the hydrochloric acid concentration in the waste hydrochloric acid is lower than 5 percent.
Owner:李绍国
Method for recycling hydrochloric acid pickling waste gas
ActiveCN101569823AAvoid wastingAvoid consumptionDispersed particle separationDirt cleaningProduction lineEconomic benefits
The invention relates to the technical field of treating hydrochloric acid pickling waste liquid-gas, in particular to a method for recycling dilute hydrochloric acid from the hydrochloric acid pickling waste liquid-gas. The method generally comprises a process of progressively downstream hydrochloric acid pickling waste gas produced by pickling lines, a process of allowing absorption liquid to progressively flow back and a process of blending and recycling. The method has the advantages that the method can recycle HCL available resources in the pickling waste gas, recover and prepare the HCL available resources into the dilute hydrochloric acid and return the dilute hydrochloric acid to production lines so as to avoid the waste of resources, and compared with a lye-spraying method, the method avoids consuming a large amount of medicament cost and has obvious economic benefit. As treated clean air is returned to the production lines as blasts so as to realize the zero-emission closed cycle of the whole waste gas treatment, waste-gas emission chimneys no longer need to be arranged outside the production lines, thus the method has strong significance for environmental protection.
Owner:WUXI XINGYI INTELLIGENT ENVIRONMENT EQUIP CO LTD
Method for producing iron oxide yellow and iron oxide red by using waste hydrochloric acid solution
The invention provides a method for producing iron oxide yellow and iron oxide red by using a waste hydrochloric acid solution. The method comprises the following steps: 1, purifying the waste hydrochloric acid solution: taking a steel waste acid solution, adjusting the pH value, adding a certain amount of waste sheet iron, carrying out controlled temperature hydrolysis for 18-24h, adding a flocculating agent, standing for above 24h, and taking the obtained supernatant; 2, preparing crystal seeds: adding the solution obtained in step 1, liquid alkali and water into a crystal seed tank, controlling the temperature and the pH value, and carrying out a preliminary oxidation reaction on ferrous chloride to generate a small amount of iron oxide nuclei; 3, carrying out an oxidation reaction: pumping the nuclei into an oxidation tank, adding sheet iron and water, and introducing compressed air to fully oxidize in order to generate iron oxide crystals on the nuclei; 4, processing byproducts; and 5, carrying out purifying post-treatment. A raw material used in the invention is the steel cleaning hydrochloric acid waste liquid, and is processed to generate useful products comprising iron oxide red and iron oxide yellow, so the waste is changed to valuables. Pigments produced through the method have the advantages of good staining degree, pure and right color, and good client feedback.
Owner:天津市大港华明化工厂
Preparation method of adsorbent resin with Cr ion cavities
InactiveCN101875003AAccurate enrichmentHigh selectivityOther chemical processesWater/sewage treatment by sorptionUltraviolet lightsPolyethylene glycol
The invention relates to a preparation method of an adsorbent resin with Cr ion cavities, belonging to the technical field of water treatment. The method comprises the following steps of: firstly, adding chitosan in an aqueous solution with ethanol, simultaneously adding single-methacrylic acid-terminated polyethylene glycol macromonomer, dual-methacrylic acid-terminated polyethylene glycol macromonomer, and then stirring to obtain a solution; secondly, adding acrylic acid or methacrylic acid in the solution, then adding Cr<3+>, and irradiating by using ultraviolet light to obtain a solid product; and thirdly, soaking the solid product in hydrochloric acid, regulating the pH of the solid product to 7 by using ammonia water, and then drying to obtain the adsorbent resin with the Cr ion cavities. The adsorbent resin prepared in the method has high selectivity and adsorbability on Cr ions, is beneficial to the accurate collection of the Cr ions to achieve the purposes of removal and recovery, and can be repeatedly used.
Owner:SHANGHAI JIAO TONG UNIV
Recycling treatment process for chlorohydric acid pickling liquid waste
InactiveCN103553256ASolve inseparableVolatileChlorine/hydrogen-chlorideWaste water treatment from metallurgical processLiquid wasteChloride
The invention discloses a recycling treatment process for chlorohydric acid pickling liquid waste. The process comprises the following steps: adjusting the acidity of CaCO3 of the chlorohydric acid pickling liquid waste to 99.65-124.56g / L; adding ferrous chloride so as to enable ferrite to be in a saturation condition; heating to the bubble point, then feeding the mixture into a rectifying tower and separating, wherein the pressure in the rectifying tower is 61.05-67.50Kpa; recovering a 10-15% diluted hydrochloric acid liquid and ferrous chloride crystals. Through reasonable parameter control and decompressing and rectifying processes, hydrochloric acid liquor applicable to acid pickling steel pieces as well as a coagulant capable of being used for sewage and wastewater treatment or a chemical raw material ferrous chloride can be recovered just by means of materials generated in the steel piece acid pickling process without spending other raw materials, so that the cost is greatly saved. The process is process-investment-saving, low in energy consumption and easy for mastering of operation, and can be continuously operated or intermittently operated in batches, thereby realizing automatically controlled operation.
Owner:YUNNAN AGRICULTURAL UNIVERSITY +1
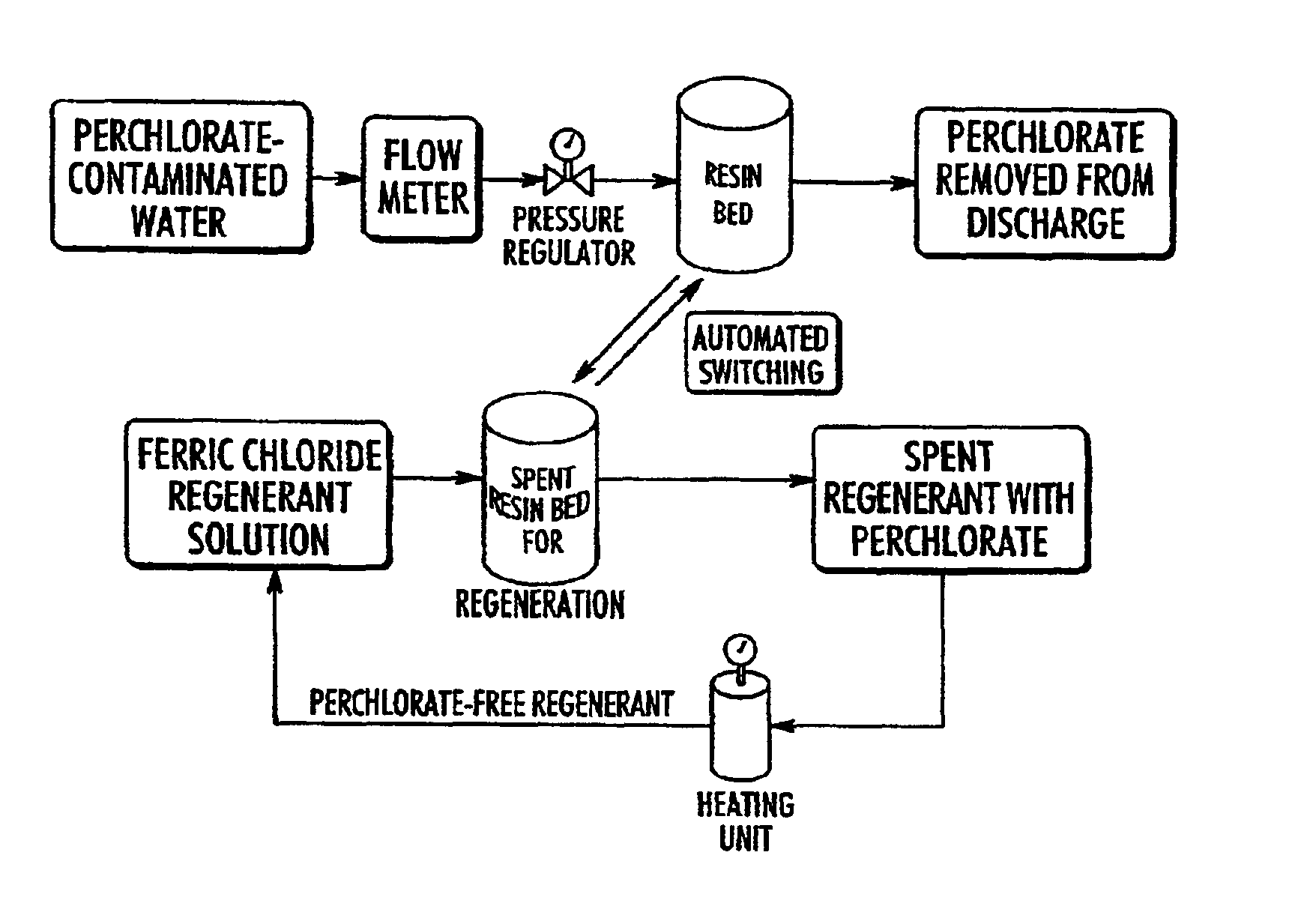
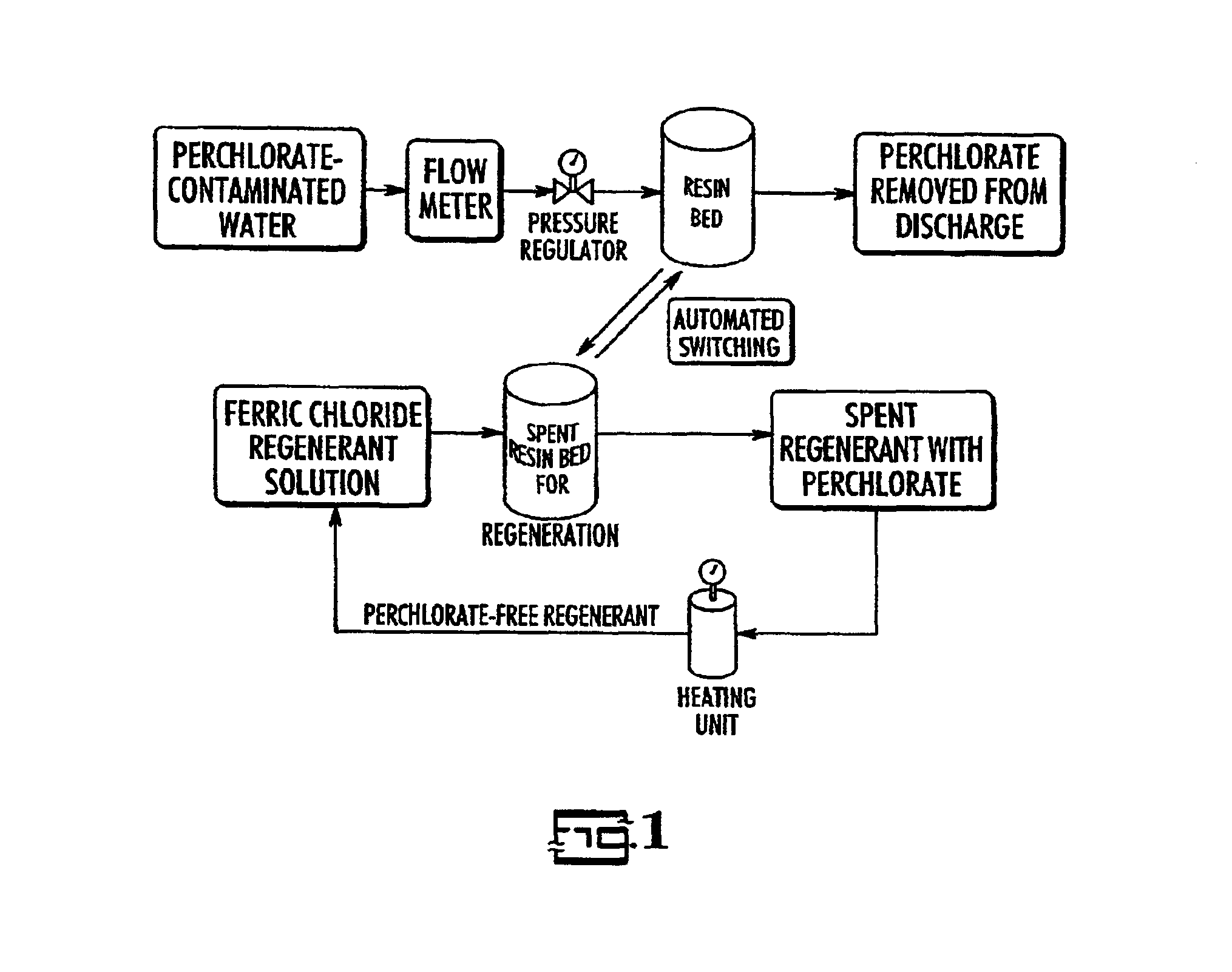
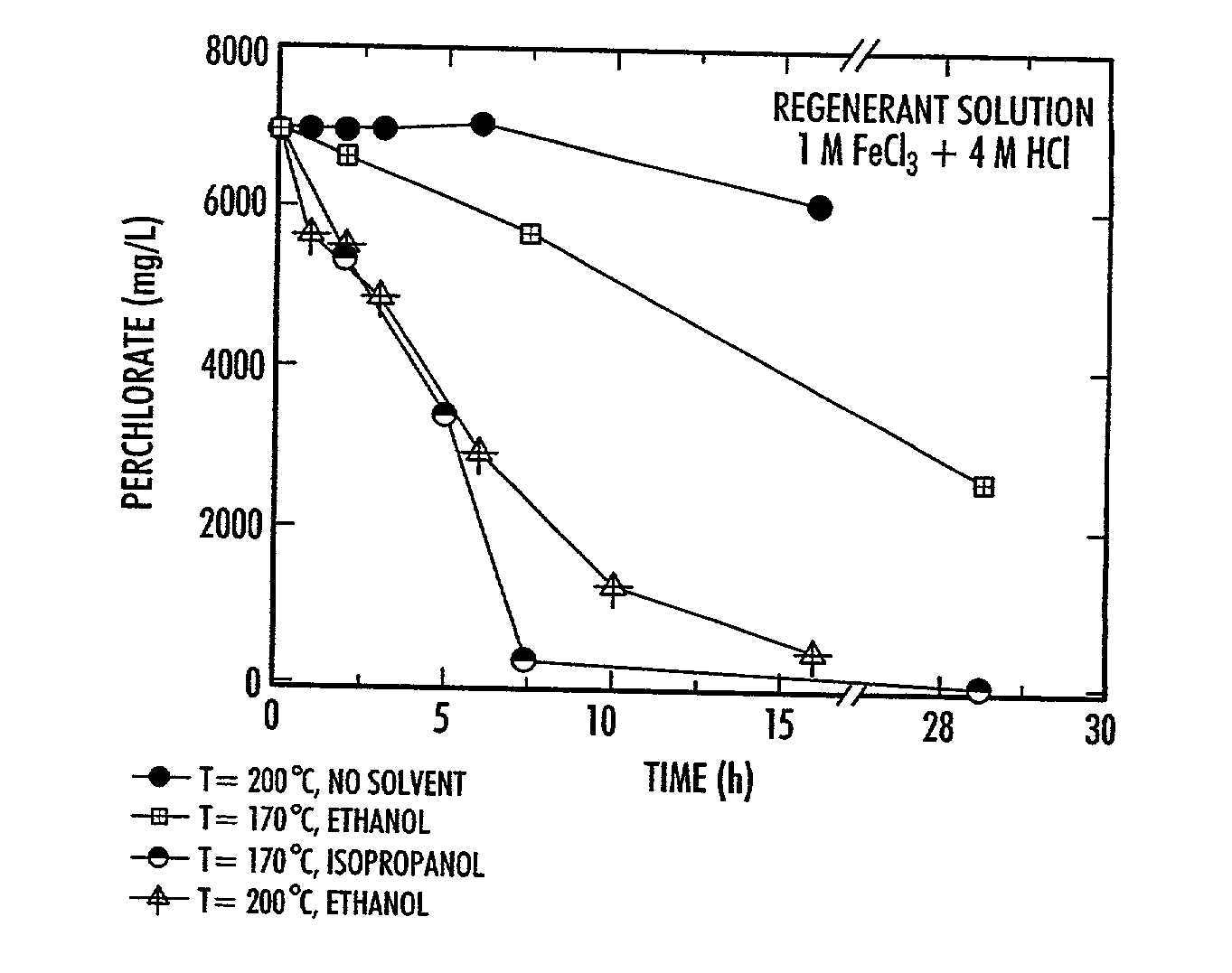
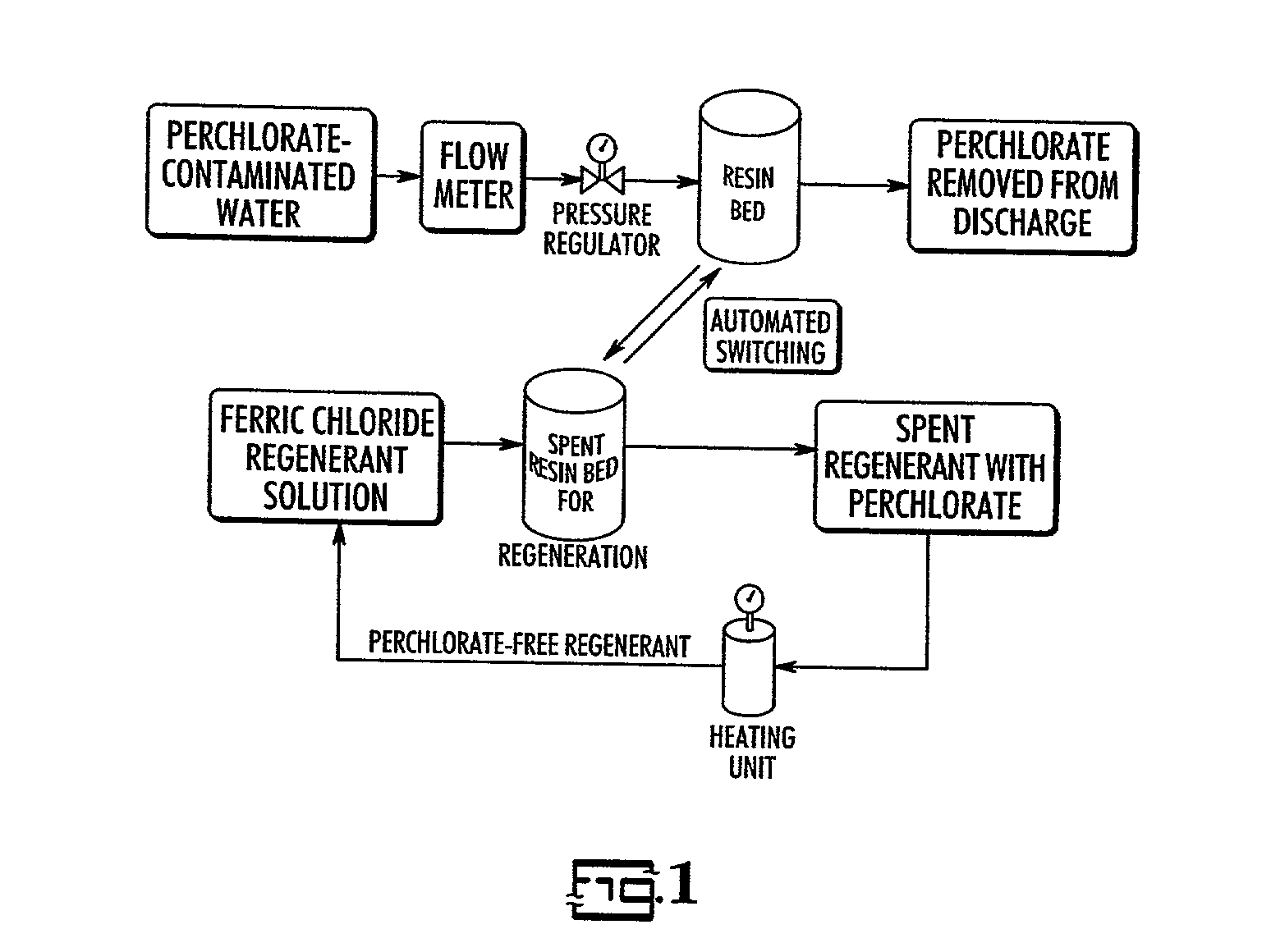
Catalytic destruction of perchlorate in ferric chloride and hydrochloric acid solution with control of temperature, pressure and chemical reagents
A method is described to decompose perchlorate in a FeCl3 / HCl aqueous solution such as would be used to regenerate an anion exchange resin used to remove perchlorate. The solution is mixed with a reducing agent, preferably an organic alcohol and / or ferrous chloride, and can be heated to accelerate the decomposition of perchlorate. Lower temperatures may be employed if a catalyst is added.
Owner:UT BATTELLE LLC
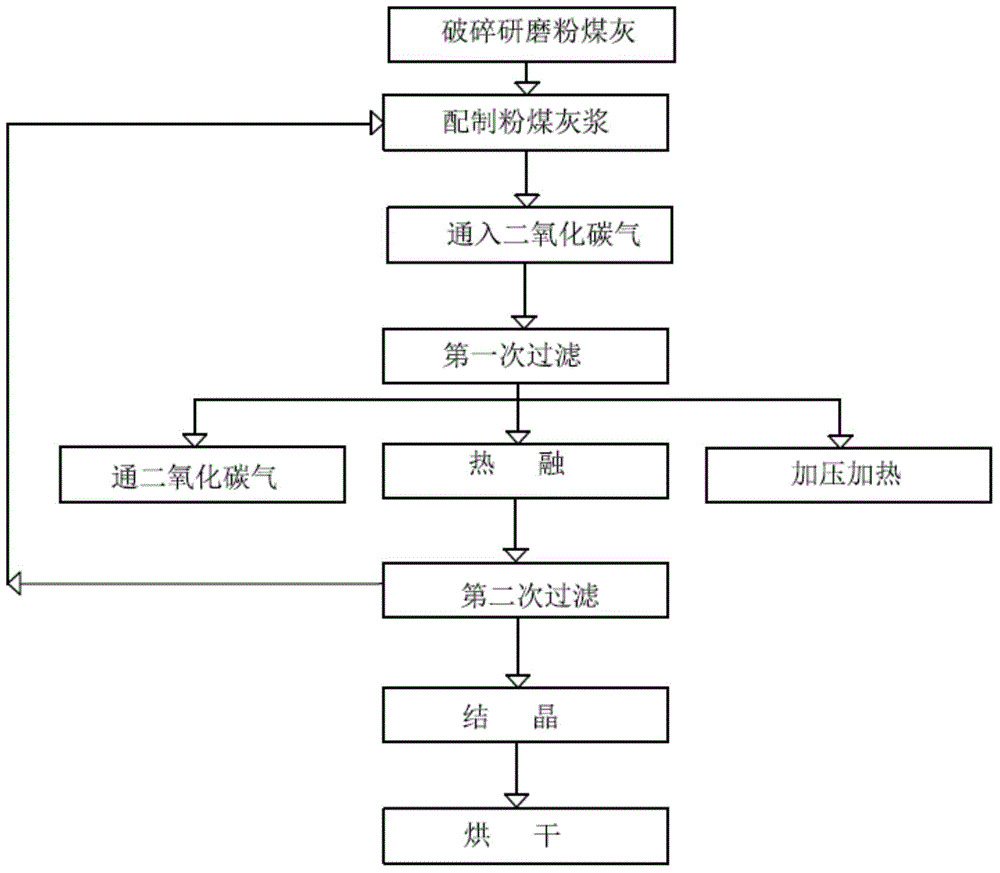
Method for extracting lithium carbonate from coal ashes
The invention relates to the technical field of preparation of lithium carbonate. A method for extracting lithium carbonate from coal ashes provided by the invention comprises the following steps: crushing and grinding coal ash filter cakes to obtain coal ash powder; putting the coal ash powder into a reaction kettle, mixing with water, and stirring evenly, so as to obtain coal ash slurry; sealing the coal ash slurry in a reaction kettle, and introducing a carbon dioxide gas, so as to obtain a lithium bicarbonate mixed liquid; filtering the lithium bicarbonate mixed liquid for the first time, so as to obtain a first filter residue and a lithium bicarbonate liquid; putting the first filter residue into hot water, and carrying out hot melting, so as to obtain a lithium carbonate mixed hot liquid; filtering the lithium carbonate mixed hot liquid to obtain a lithium carbonate hot liquid and a second filter residue; mixing the lithium bicarbonate liquid with the lithium carbonate hot liquid, and carrying out crystallizing treatment, so as to obtain lithium bicarbonate crystal and a lithium bicarbonate liquid; adding alkali carbonate or a dilute hydrochloric acid liquid to the lithium bicarbonate liquid, and filtering to obtain a filtered material; and calcining the filtered material and lithium carbonate crystal, so as to obtain lithium carbonate.
Owner:宋英宏
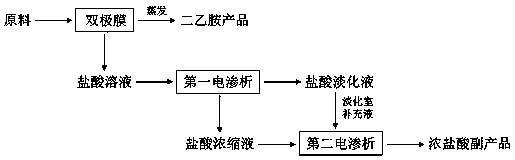
Clean diethylamine production method
InactiveCN108017121ARealization of clean production and zero emissionAchieve emissionsGeneral water supply conservationWater contaminantsResource recoveryPhysical chemistry
The invention belongs to the field of industrial sewage disposal and relates to a clean diethylamine production method. According to the clean diethylamine production method for zero emission and resource recycle, through bipolar membranes in a bipolar membrane device, a diethylamine alkali liquid and a hydrochloric acid solution are obtained, the diethylamine alkali liquid is further subjected toevaporation treatment to obtain a product in which the content of diethylamine alkali reaches 99.0% or above, and the content of chloride ions is lower than 0.1%; the hydrochloric acid solution obtained from the bipolar membrane device is disposed by a first electrodialysis device to obtain a hydrochloric acid concentrated solution and a hydrochloric acid desalted solution; the hydrochloric acidconcentrated solution obtained from the first electrodialysis device is disposed by a second electrodialysis device to obtain a side product, namely a concentrated hydrochloric acid solution; the hydrochloric acid desalted solution obtained from the first electrodialysis device serves as a replenisher of a desalting chamber of the second electrodialysis device. Therefore, the method can not only achieve clean production of diethylamine but also have great social and economic benefits at the same time.
Owner:浙江迪萧科技有限公司
Catalytic destruction of perchlorate in ferric chloride and hydrochloric acid solution with control of temperature, pressure and chemical reagents
A method is described to decompose perchlorate in a FeCl3 / HCl aqueous solution such as would be used to regenerate an anion exchange resin used to remove perchlorate. The solution is mixed with a reducing agent, preferably an organic alcohol and / or ferrous chloride, and can be heated to accelerate the decomposition of perchlorate. Lower temperatures may be employed if a catalyst is added.
Owner:UT BATTELLE LLC
Preparation method of nano TiO2 film modified by metal oxide
InactiveCN103007916AHigh photocatalytic degradation rateThe reaction process is easy to controlMetal/metal-oxides/metal-hydroxide catalystsPlasma electrolytic oxidationMicro arc oxidation
The invention discloses a preparation method of a nano TiO2 film modified by metal oxide. The method comprises the following steps: firstly, a pure titanium sheet is prepared into 25mm*15mm*2mm rectangular sheet; secondly, the titanium sheet is conducted to pretreatment, and pretreatment comprises sanding, oil removing, hydrochloric acidizing fluid rinsing, ultrasonic deionized water cleaning and vacuum drying; thirdly, 2 to 20mmol metal oxide required to be conducted to doping is respectively coated on the surface of the pretreated titanium sheet through heated viscous polyvinyl alcohol, after drying, and the titanium sheet is directly arranged on micro-arc oxidation equipment to be conducted to micro-arc oxidation, wherein the titanium sheet is served as a positive electrode to be dipped in treating fluid, a stainless steel container with a cooling system is served as a negative electrode, and experiment parameters are as follows: the voltage is 420V, the preparation time is 30min, the content of Na3PO4 in a phosphate electrolyte system is 10g / L, the content of NaF is 1g / L, and the temperature of electrolyte is controlled to be lower than 35 DEG C; and fourthly, a prepared nano TiO2 film is conducted to deionized water rinsing and drying. The nano TiO2 film has high photocatalytic degradation ratio, and is beneficial to industrial production.
Owner:CHANGZHOU UNIV
Treatment method of broken materials of solar silicon cell pieces
InactiveCN102070146AGood removal effectMeet cleaning requirementsSilicon compoundsHydrofluoric acidMegasonic cleaning
The invention discloses a treatment method of broken materials of solar silicon cell pieces, and relates to the treatment method of the broken materials of the solar silicon cell pieces. The method comprises the following steps in sequence: a. the broken materials of the cell pieces are put into hydrofluoric acid liquid and soaked for 10-15 hours; b. then the broken materials are soaked in mixed liquid of hydrofluoric acid and ammonium fluoride, wherein the mixed liquid comprises the following components in percentage by weight: the ratio of the hydrofluoric acid and the ammonium fluoride is equal to 65-90%:35-10%; c. the broken materials are put into hydrofluoric acid liquid and soaked; d. the broken materials are put into mixed liquid of nitric acid, hydrochloric acid and hydrofluoric acid and soaked; e. the broken materials are arranged into sodium hydroxide solution; f. the broken materials are put into an ultrasonic cleaning groove for cleaning; and g. the broken materials are dewatered and put into a baking oven for drying. The method has the beneficial effects that compared with the traditional treatment method, metals on the broken materials of the cell pieces are better removed, the mass conforms to the cleaning requirements of the solar silicon industry completely, the quality is ensured, and the availability ratio of raw materials is increased; and after cleaning, waste acid and rinsing liquid are treated and then discharged, so no pollution is caused to the environment.
Owner:KAIFENG WANSHENGXIN MATERIALS
Method for treating waste brine sludge of soda by hydrochloric acid
InactiveCN101823822AEliminate chemical pollutionSimple production processWater/sewage treatment by centrifugal separationSludge treatmentCalcium hydroxideSludge
Owner:汪晋强
Method for preparing flotation collector containing gold mine sulfide
The invention provides a method for preparing a flotation collector containing gold mine sulfide. The method comprises the following steps of: adding 300 to 500 ml of n-decene and 30 to 50 g of aluminum trichloride catalyst into a 1L high pressure reaction kettle provided with a thermometer, a stirrer, a gas inlet pipe and a gas outlet pipe, keeping the temperature of 50 DEG C, continuously stirring the mixture, discontinuously filling hydrogen sulfide gas in the reaction kettle, maintaining the pressure in the kettle to 2 to 3 MPa, washing the reaction product by hydrochloric acid solution and solution of sodium hydroxide after 3 to 5h of reaction, separating an organic phase for decompression rectification, and collecting a fraction at the temperature of between 180 and 230 DEG C under the normal pressure, which is the flotation collector containing gold mine sulfide. When the flotation collector containing gold mine sulfide is used, an activator is not or less needed, and the total recovery of the gold-containing sulfide ores reaches more than 90 percent, and the production cost is lowered.
Owner:GUANGXI UNIV
Preparation method for metal oxide modified nano-TiO2 film material
InactiveCN103041798AHigh photocatalytic degradation rateThe reaction process is easy to controlCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsElectrolysisPlasma electrolytic oxidation
The invention discloses a preparation method for a metal oxide modified nano-TiO2 film material. The method comprises the following steps: (1) a pure titanium sheet is prepared to a rectangular sheet with dimensions of 25 mm*15 mm*2 mm; (2) the titanium sheet is pretreated through sanding, oil removing, hydrochloric acid liquid rinsing, ultrasonic de-ionized water cleaning and vacuum drying; (3) the pretreated titanium sheet is placed on micro-arc oxidization equipment; the experiment parameters are as follows: the voltage is 420 V; the preparation time is 30 min; the concentration of Na3PO4 in a phosphate electrolyte system is 10 g / L; the concentration of NaF is 1 g / L; the electrolytic temperature is controlled below 35 DEG C; then 2-20 mmol metal oxide is added in the electrolyte, and is reacted under the magnetic stirring so as to synchronously implement the doping and the micro-arc oxidization, wherein the titanium sheet as anode is dipped in treating agent; and a stainless steel vessel provided with a cooling system is used as cathode; and (4) the prepared nano-TiO2 film is rinsed by de-ionized water and is dried. The nano-TiO2 film prepared by the method has high photocatalytic degradation rate, and brings convenience to industrial production.
Owner:CHANGZHOU UNIV
Processing method of coating film crushed materials of solar silicon cell
InactiveCN102151669AAlleviate material shortageImprove availabilityCleaning using liquidsSilicon cellSodium hydroxide
The invention relates to a processing method of coating film crushed materials of a solar silicon cell. The processing method sequentially comprises the following steps of: a, placing the coating film crushed materials into a hydrofluoric acid solution for soaking; b, placing into a hydrochloric acid solution for the soaking; c, placing into a mixed solution of nitric acid, hydrochloric acid and hydrofluoric acid for soaking; d, placing into a sodium hydroxide solution for soaking; e, placing into an ultrasonic cleaning tank for cleaning; and f, dewatering after ultrasonic cleaning and then placing into a drying oven for drying. Compared with the traditional processing method, the processing method has the remarkable effects of completely meeting the cleaning requirements of a solar silicon industry on the quality and ensuring the quality, thereby realizing the reuse of waste materials and enhancing the availability of raw materials; and after cleaning is completed, waste acids and bleaching liquor are treated and then drained without polluting environment.
Owner:KAIFENG WANSHENGXIN MATERIALS
Non-organic solvent extraction method for chenodeoxycholic acid in bird gall
InactiveCN101143886AReduce extraction production costsLess investmentSteroidsBird material medical ingredientsChenodeoxycholic acidSalt deposit
The present invention discloses an extraction method of the inorganic solvent of chenodeoxycholic acid in the avian bile. The first step is a saponification reaction, that is, the avian bile and sodium hydroxide are boiled and saponified according to a certain proportion, and the saponification lasts twenty to twenty four hours; the second step is a counteraction reaction, that is, saponification solution is counteracted by hydrochloric acid, the pH value is regulated to two to four, and after deposition, total bile acid is generated; the third step is the calcium salt deposition, that is, the total bile acid is resolved in oxyhydrogen sodium solution, and after the addition of activated carbon, refluence and filtration, the calcium salt deposit is obtained by adding ten to fourteen percent of calcium chloride into filtrate; the fourth step is decalcification, that is, sodium carbonate is added into calcium salt according to a proportion between ten percent and fifteen percent, heating and refluence last one to three hours, and the pH value of filtrate is regulated to 2.0 to 3.5 by five to ten percent of hydrochloric solution, so that deposit is obtained; the fifth step is the collection of the deposit, that is, the crude chenodeoxycholic acid is generated by vacuum drying. Ethanol and 120 # gasoline are not needed, the investment of equipment is reduced, the process cycle is shortened, and the cost of extraction is reduced.
Owner:刘永存
Process for recycling iron/steel acid-washing waste hydrochloric acid through evaporation burning and coupling process
InactiveCN101786606AEfficient separationIncrease the number ofChlorine/hydrogen-chlorideFluid phaseWater chlorination
The invention discloses a process for recycling iron / steel acid-washing waste hydrochloric acid through an evaporation burning and coupling process, which belongs to the technology of iron / steel acid-washing waste hydrochloric acid recycling packaged process. In the process: first, 50-70 percent of water and free hydrochloric acid in the waste hydrochloric acid are vaporized and separated after being heated and evaporated, the vapor is separated from the waste hydrochloric acid, and the proportion of the waste acid reaches 1.4-1.6g / cm<3>; the dilute hydrochloric acid is obtained through vapor condensation; and the liquid phase adopts a thermal crystallization process, the evaporation temperature is controlled within 100-110 DEG C, azeotropic hydrochloric acid is obtained by evaporation from the concentrated waste hydrochloric acid for the second time, the ferrous chloride is uninterruptedly and continuously separated from the waste acid concentrated solution, and the ferrous chloride is crystallized; second, the obtained ferrous chloride is burnt under 800 DEG C to obtain ferric oxide powder and HCl gas; the gas is absorbed for stages by the dilute hydrochloric acid obtained by adopting the evaporation process and the thermal crystallization process, and finally, the hydrochloric acid with different concentrations can be obtained to directly return to the acid-washing line for reuse. The invention recycles and reuses the acid-washing waste acid through the process, thereby reducing the consumption of the iron / steel acid-washing waste hydrochloric acid, being beneficial for the environmental protection and realizing the purposes of recycling and reusing the waste hydrochloric acid.
Owner:BEIJING TIANTAIXING ENG TECH
Method for preparing solar grade silicon from silica serving as raw material
InactiveCN101935846AReduce pollutionEfficient removalElectrolysis componentsSilicon compoundsOxygenVacuum evaporation
The invention provides a method for preparing solar grade silicon from silica serving as a raw material. In the method, the silica serves as the raw material, and CaCl2 is taken as a molten salt electrolyte; and the method comprises the following steps of: reducing the silica at the temperature of between 600 and 1,000 DEG C by using the molten salt electrolyte to directly electrolytically reduce silicon in the silica into elementary silicon; adding a compound into the elementary silicon according to a mass ratio of 1:3-9, and introducing protective gas at the temperature of between 600 and 1,200 DEG C for crystallization; putting the crystalized silicon into hydrochloric acid liquid with the concentration of between 1 and 5 mol / L according to a solid-liquid mass ratio of 1:1-8, and performing acid leaching for 2 to 48 hours; washing by using water for 2 to 5 times, and drying at the temperature of between 40 and 150 DEG C; melting the dried silicon at the temperature of between 1,450 and 1,650 DEG C, and performing vacuum evaporation under the vacuum condition at the temperature of between 1,600 and 1,650 DEG C and under the pressure of between 1*10<-2> and 1*10<10> Pa; and condensing the evaporated melt silicon at a condensation speed of between 0.1 and 5 mm / h to prepare the solar grade silicon. By reducing to remove impurities such as oxygen, phosphorus, aluminum, calcium, titanium and the like, and through high temperature crystallization and acid leaching for removing boron, the product has the purity of over 99.99991 percent, and the method has the advantages of short production flow, low cost and light environment pollution.
Owner:KUNMING UNIV OF SCI & TECH
Catalyst for preparing bio-diesel, preparation method and application thereof
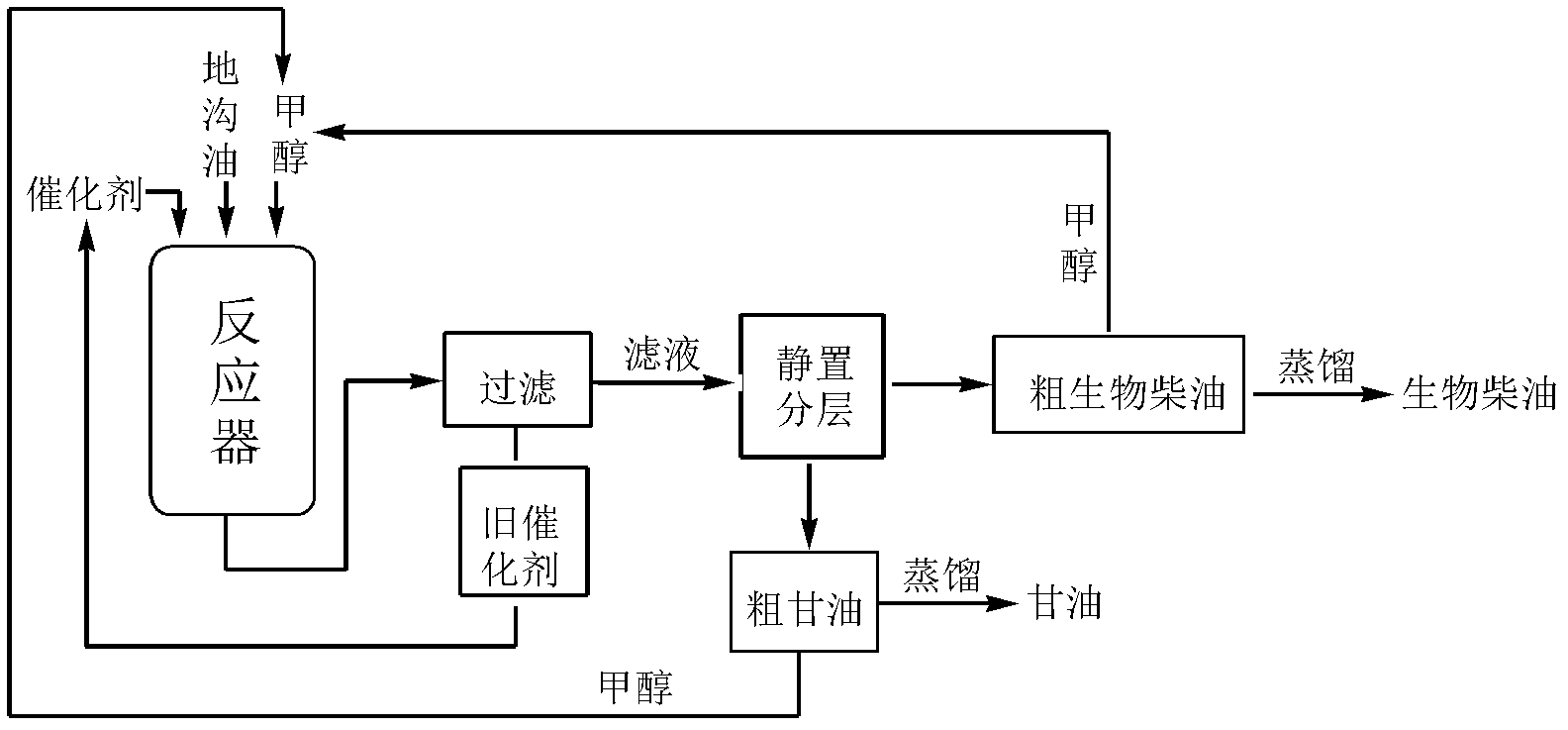
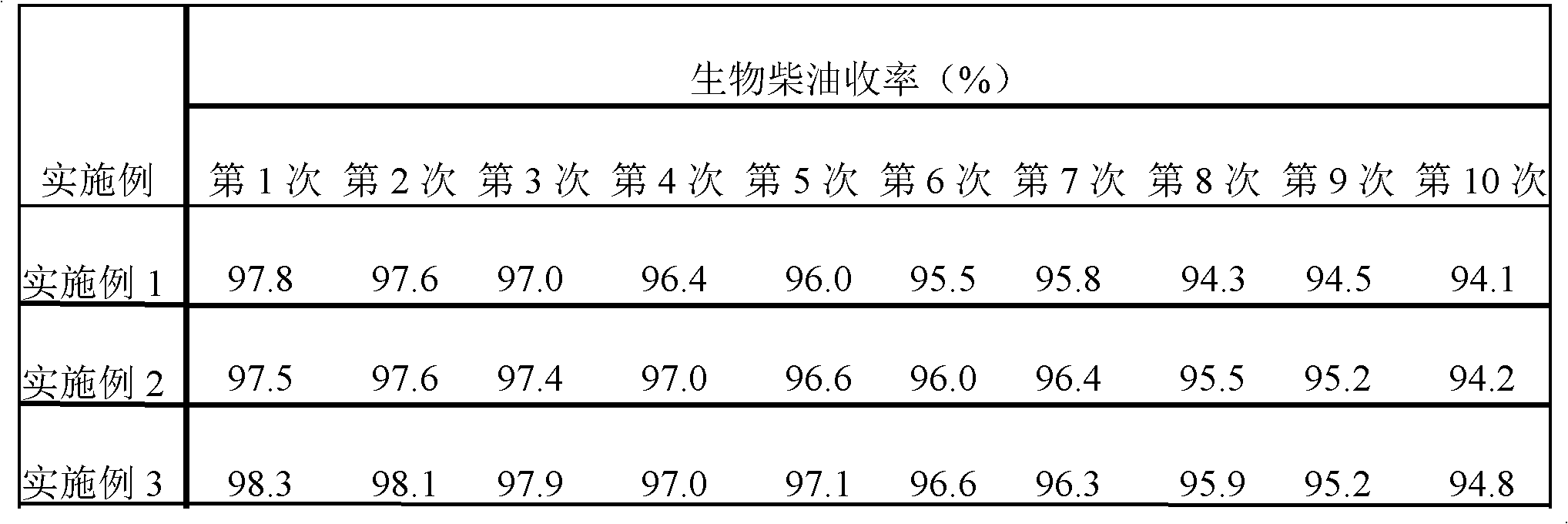
InactiveCN102430427AEasy to prepareRaw materials are easy to getOrganic-compounds/hydrides/coordination-complexes catalystsBiofuelsBiodieselReaction temperature
The invention discloses a catalyst for preparing bio-diesel, which is obtained by the following preparation method: dripping the hydrochloric acid liquid of ammonium persulphate to the hydrochloric acid liquid of aniline; stirring at 10-18 DEG C for 3-4 hours; filtering, washing the solid till to neutral, and drying; soaking the solid after being dried in toluenesulfonic acid liquid for 20-24 hours, filtering and drying to obtain the catalyst doped poly-aniline in the toluenesulfonic acid. The catalyst has catalytic action while the bio-diesel is prepared with the drainage oil. Compared with the prior art, the invention has the following advantages: (1) the catalyst has high catalytic activity while the bio-diesel is prepared with the drainage oil, small quantity of the catalyst, short reaction time, low reaction temperature and high yield of the product which is 97.5-98.9%; (2) the catalyst preparation method is simple, the price is low, the catalyst after being used can be directly reused without any treatment and can be reused for many times, and the catalytic activity is changed.
Owner:LIAOCHENG UNIV
Treatment recycling process of hydrochloric acid liquid waste produced in rare metals extraction process
InactiveCN102616743ARealize refining and recyclingSave heatChlorine/hydrogen-chloride purificationLiquid wasteEvaporation
The invention discloses a treatment recycling process of hydrochloric acid liquid waste produced in a rare metals extraction process, which belongs to the technical field of hydrochloric acid liquid waste treatment. The treatment recycling process adopts a multiple-effect evaporation mode to achieve evaporation of the hydrochloric acid liquid waste, greatly saves heat energy, achieves extraction recycling of products, simultaneously guarantees reduction of production cost, achieves recycling utilization of heavy metal elements in the hydrochloric acid liquid waste and solves the problem of environmental protection.
Owner:常熟市新港农产品产销有限公司
Porous brick, hollow brick prepared from waste hydrochloric acid liquid containing iron and its preparation method
The invention discloses a porous brick and hollow brick with waste ferric alcaine liquid, which comprises the following parts: 30-50% clay, 40-60% shale, 10-20% coal slag, inferior coal, coal ash, coal waste or their mixture, 1-3% waste of ferric ore, wherein the particle of each material is less than 1mm. The preparing method comprises the following steps: stirring waste ferric alcaine liquid and supernatant reacted by waste iron filling and ferric ore waste and all components completely; blending evenly; aging; compressing; stirring; squeezing; cutting mould; piling mould; stewing; drying; sintering; cooling naturally.
Owner:王铁林
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com