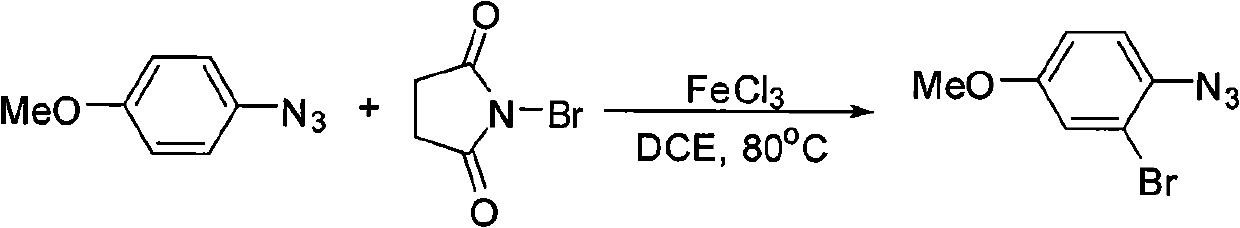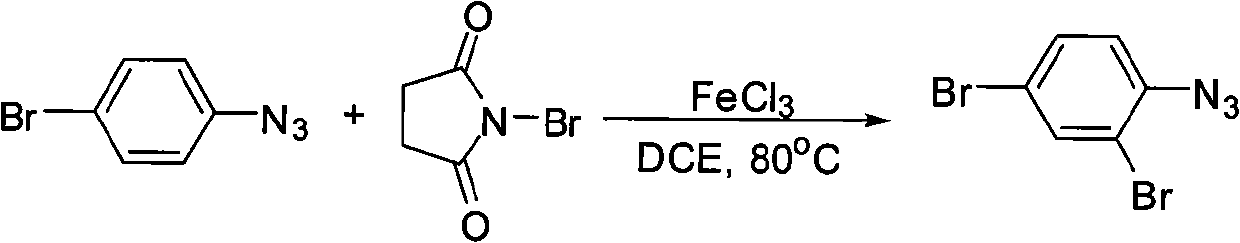Method for preparing bromoaryl azide
A technology of aryl azide and compound, which is applied in the field of preparation of bromoaryl azide compound, can solve the problems of small application range and achieve the effects of low cost, mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: Preparation of 2,4-dibromophenylazide
[0017]
[0018] 3 mL of 1,2-dichloroethane, 16 mg (0.1 mmol) of anhydrous ferric chloride, 89 mg (0.5 mmol) of NBS, and 99 mg (0.5 mmol) of p-bromoazide were added to a 60 mL sealed tube, and the mixture was stirred at 80°C. The reaction was carried out for 24 hours, and 2 mL of water was added to the reaction solution, followed by extraction with ethyl acetate. After the organic layer was washed with saturated brine and dried over anhydrous sodium sulfate, the solvent was removed by distillation under reduced pressure to obtain a crude product. The crude product was separated by column chromatography (petroleum ether as the eluent) to obtain 2,4-dibromophenyl stack. Nitrogen 84 mg, pale yellow liquid, yield 61%.
[0019] Its NMR data are as follows:
[0020] 1 H NMR (500MHz, CDCl 3 ): δ=7.04 (1H, d, J=8.5Hz), 7.46 (1H, dd, J=8.5, 2.1Hz), 7.70 (1H, d, J=2.1Hz).
Embodiment 2
[0021] Example 2: Preparation of 2,4-dibromophenylazide
[0022]
[0023] 3 mL of 1,2-dichloroethane, 32 mg (0.2 mmol) of anhydrous ferric chloride, 98 mg (0.55 mmol) of NBS, and 99 mg (0.5 mmol) of p-bromoazide were added to a 60 mL sealed tube, and the reaction was stirred at 80°C. After 24 hours, 2 mL of water was added to the reaction solution, followed by extraction with ethyl acetate. After the organic layer was washed with saturated brine and dried over anhydrous sodium sulfate, the solvent was removed by distillation under reduced pressure to obtain a crude product. The crude product was separated by column chromatography (petroleum ether as the eluent) to obtain 2,4-dibromophenyl stack. Nitrogen 108 mg, pale yellow liquid, yield 78%.
[0024] Its NMR data are as follows:
[0025] 1 H NMR (500MHz, CDCl 3 ): δ=7.04 (1H, d, J=8.5Hz), 7.46 (1H, dd, J=8.5, 2.1Hz), 7.70 (1H, d, J=2.1Hz).
Embodiment 3
[0026] Example 3: Preparation of ethyl 3-bromo-4-azidobenzoate
[0027]
[0028] 3 mL of 1,2-dichloroethane, 32 mg (0.2 mmol) of anhydrous ferric chloride, 98 mg (0.55 mmol) of NBS, 96 mg (0.5 mmol) of ethyl 4-azidobenzoate were added to a 60 mL sealed tube, and the mixture was heated to 80 The reaction was stirred at °C for 24 hours, and 2 mL of water was added to the reaction solution, followed by extraction with ethyl acetate. After the organic layer was washed with saturated brine and dried over anhydrous sodium sulfate, the solvent was removed by distillation under reduced pressure to obtain a crude product. The crude product was separated by column chromatography (petroleum ether as eluent) to obtain 3-bromo-4-azidobenzene Ethyl formate, 109 mg, was a pale yellow liquid, and the yield was 81%.
[0029] Its NMR data are as follows:
[0030] 1 H NMR (500MHz, CDCl 3 ): δ=1.40 (3H, t, J=7Hz), 4.38 (2H, q, J=7Hz), 7.21 (1H, d, J=8.5Hz), 8.02 (1H, dd, J=8.5, 1.5Hz) ), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com