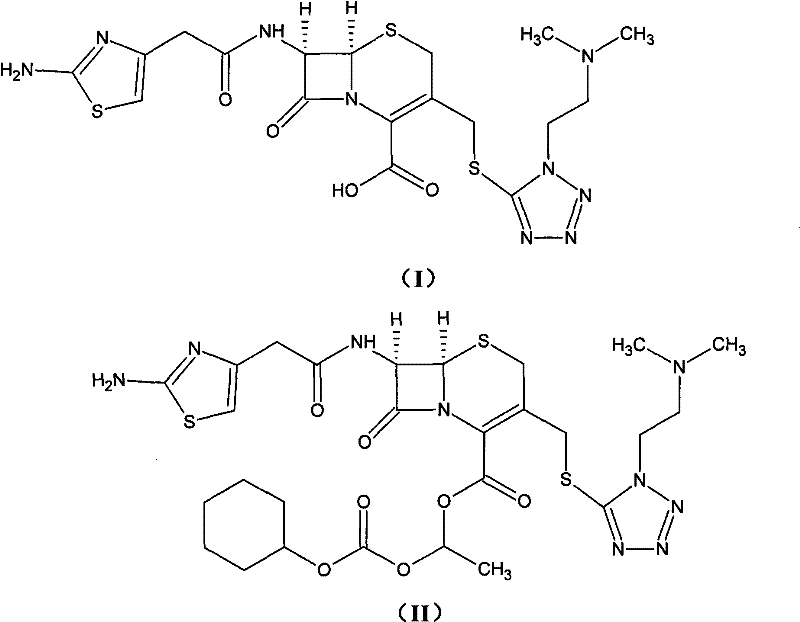
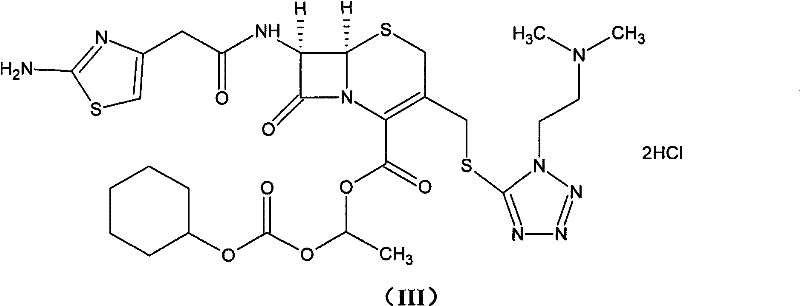
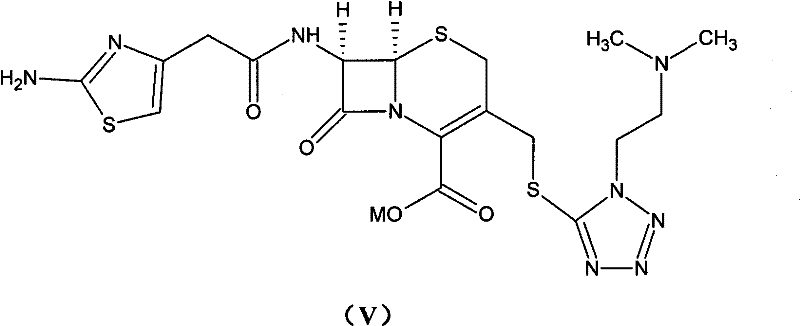
Method for preparing cefotiam hexetil hydrochloride
A technology of cefotiam and cefotiam, which is applied in the field of drug antibiotics and can solve the problems of increasing crystallization loss and increasing separation processes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] (1) Add 600L of acetonitrile and 140Kg of pre-dried NaI to the reaction tank, heat to 50°C under stirring, add 130L of 1-chloroethylcyclohexyl carbonate, and react for 100min. After the reaction was completed, it was concentrated to dryness under reduced pressure at room temperature. CH 2 Cl 2 600L and Na 2 S 2 o 3 Aqueous solution (5%) 400L mixed solvent washes concentrate, collects organic layer, obtains the CH of 1-iodide ethyl cyclohexyl carbonate 2 Cl 2 solution.
[0068] (2) Add KHCO to the reaction tank 3 100Kg, water 30L, acetone 60L and stir to dissolve. Then cefotiam 130Kg was added and stirred at 28°C for 40min. Add 1400L of acetone and stir to dissolve, then cool to 0°C, let stand to separate layers and collect the lower layer, then add DMA600L, stir and dissolve at 25°C to obtain cefotiam salt solution.
[0069] (3) CH of 1-iodoethyl cyclohexyl carbonate 2 Cl 2 The solution was added to the cefotiam salt solution and stirred for reaction at a te...
Embodiment 2
[0075] (1) Add 600L of acetonitrile and 140Kg of pre-dried NaI to the reaction tank, heat to 50°C under stirring, add 130L of 1-chloroethylcyclohexyl carbonate, and react for 100min. After the reaction was completed, it was concentrated to dryness under reduced pressure at room temperature. CH 2 Cl 2 600L and Na 2 S 2 o 3 Aqueous solution (5%) 400L mixed solvent washes concentrate, collects organic layer, obtains the CH of 1-iodide ethyl cyclohexyl carbonate 2 Cl 2 solution.
[0076] (2) Add KHCO in the reaction tank 3 100Kg, water 30L, acetone 60L and stir to dissolve. Then cefotiam 130Kg was added and stirred at 28°C for 40min. Add 1700L of acetone and stir to dissolve, then cool to 0°C, let stand to separate layers and collect the lower layer, then add DMA600L, stir and dissolve at 25°C to obtain cefotiam salt solution.
[0077] (3) CH of 1-iodoethyl cyclohexyl carbonate 2 Cl 2 The solution was added to the cefotiam salt solution and stirred for reaction at a temp...
Embodiment 3
[0083] (1) Add 600L of acetonitrile and 140Kg of pre-dried NaI to the reaction tank, heat to 50°C under stirring, add 130L of 1-chloroethylcyclohexyl carbonate, and react for 100min. After the reaction was completed, it was concentrated to dryness under reduced pressure at room temperature. CH 2 Cl 2 600L and Na 2 S 2 o 3 Aqueous solution (5%) 400L mixed solvent washes concentrate, collects organic layer, obtains the CH of 1-iodide ethyl cyclohexyl carbonate 2 Cl 2 solution.
[0084] (2) Add KHCO to the reaction tank 3 100Kg, water 30L, acetone 60L and stir to dissolve. Then cefotiam 130Kg was added and stirred at 28°C for 40min. Add 1700L of acetone and stir to dissolve, then cool to 0°C, let stand to separate layers and collect the lower layer, then add DMA600L, stir and dissolve at 25°C to obtain cefotiam salt solution.
[0085] (3) CH of 1-iodoethyl cyclohexyl carbonate 2 Cl 2 The solution was added to the cefotiam salt solution and stirred for reaction at a te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com