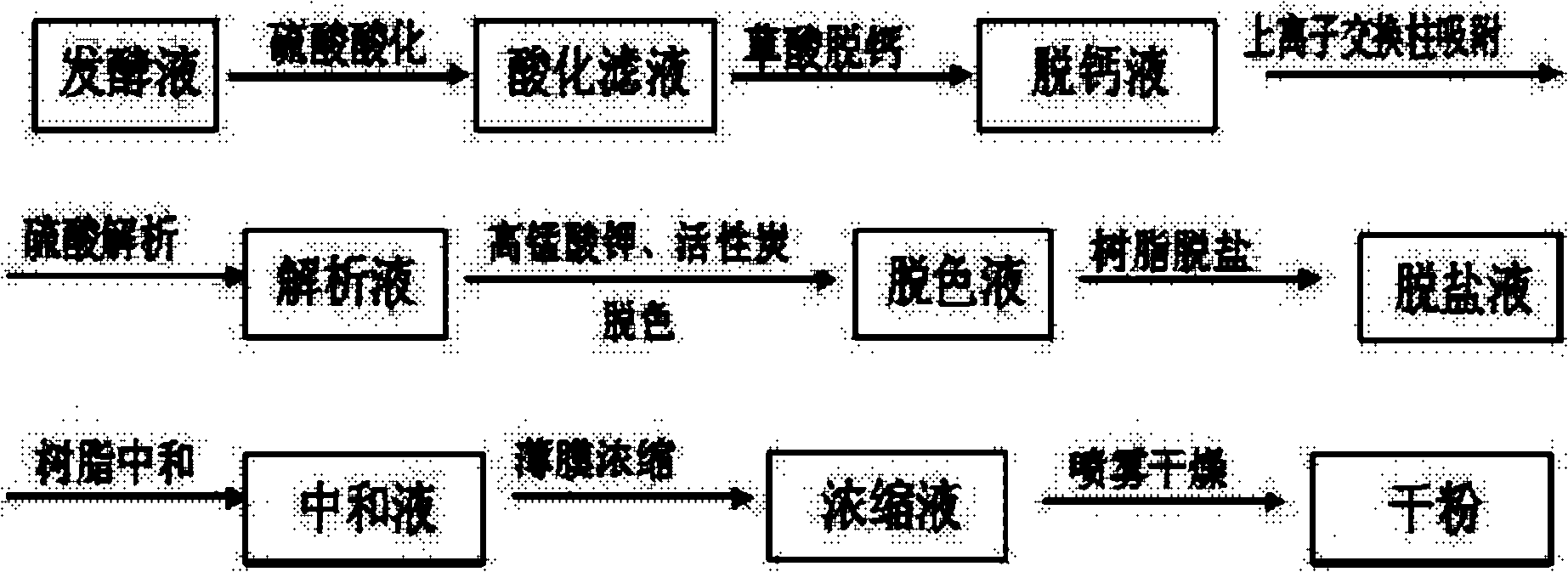
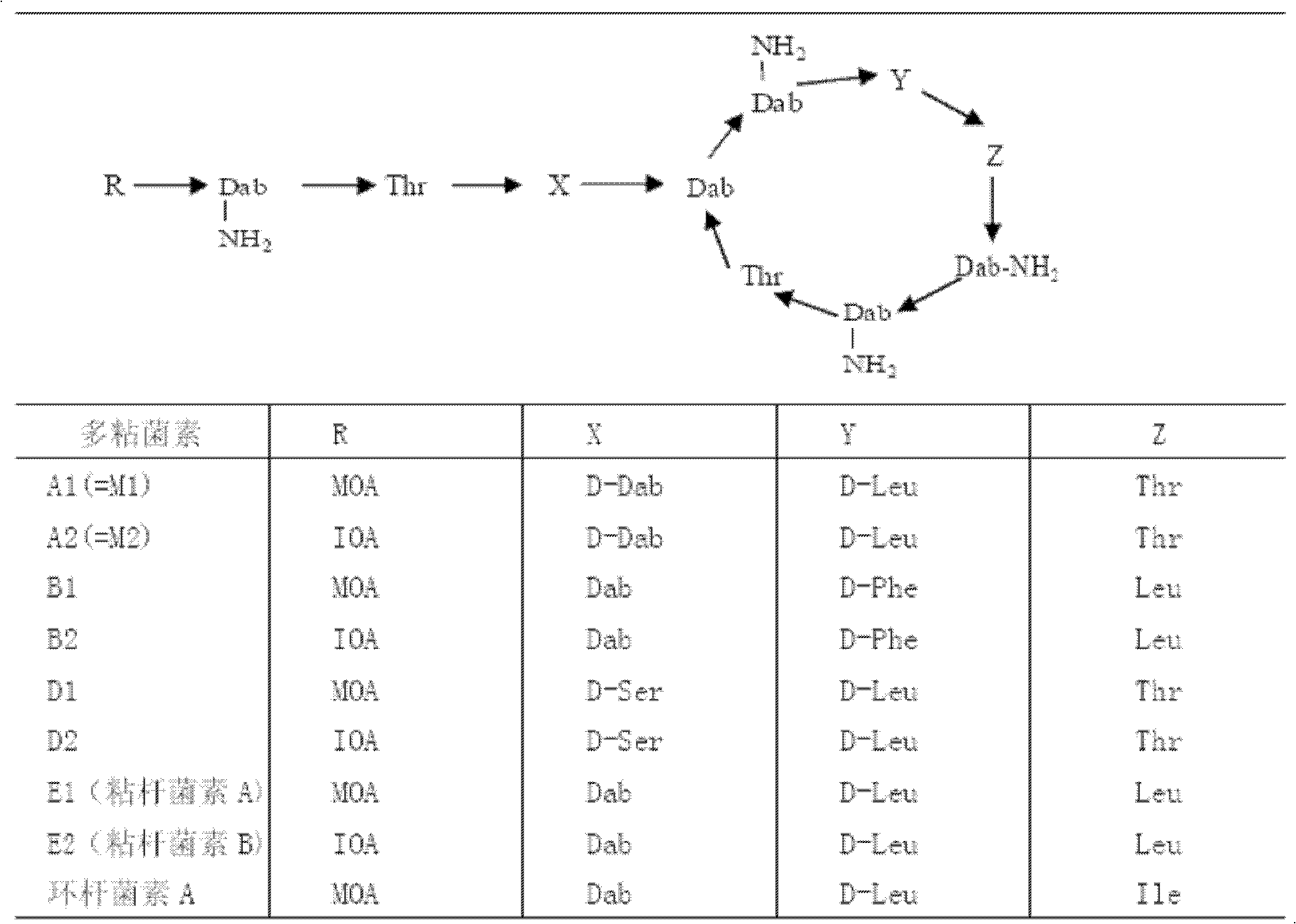
Method for improving total component content of colistin sulphate
A technology of colistin sulfate and component content, which is applied in the field of medicine, can solve the problems of low purity and achieve the effects of improving purity, simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Colistin sulfate dry powder (prepared according to the ion exchange method mentioned in the background technology of the present invention, commercially available product) 140g, detected by HPLC normalization method, its total component content is 72.64%, add pure water to dissolve to 550ml, then Add 350ml of acetone while stirring, and let stand to separate layers. After 1 hour, separate the supernatant and the lower layer of precipitate with a separatory funnel. Add water to the lower layer of precipitate to 680ml before it is completely dissolved. Take about 10ml of dilution and detect it by HPLC normalization method. Its total component content is 77.69%;
[0029]For the remaining 670ml, 240ml of acetone was added while stirring, and the layers were allowed to stand. After 1 hour, the supernatant was separated from the lower precipitate with a separatory funnel, and the lower precipitate was vacuum-dried at 80°C for 4 hours to obtain 106.7g of colistin sulfate dry po...
Embodiment 2
[0031] Colistin sulfate dry powder (prepared according to the ion exchange method mentioned in the background technology of the present invention, commercially available product) 150g, detected by HPLC normalization method, its total component content is 71.79%, add pure water to dissolve to 590ml, then Add 180ml of ethanol while stirring, and let stand to separate layers. After 1 hour, separate the supernatant and the lower layer of sediment with a separatory funnel. Add water to the lower layer of precipitate to 650ml before it is completely dissolved. Take about 10ml of dilution and detect it by HPLC normalization method. Its total component content is 78.13%;
[0032] For the remaining 640ml, 120ml of ethanol was added while stirring, and the layers were allowed to stand. After 1 hour, the supernatant was separated from the lower precipitate with a separatory funnel, and the lower precipitate was vacuum-dried at 80°C for 4 hours to obtain 126.7g of colistin sulfate dry powd...
Embodiment 3
[0034] Colistin sulfate dry powder (prepared according to the ion exchange method mentioned in the background technology of the present invention, commercially available product) 120g, detected by HPLC normalization method, its total component content is 72.77%, add pure water to dissolve to 550ml, then Add 270ml of isopropanol while stirring, let it stand for stratification, and separate the supernatant and the lower layer of precipitate with a separatory funnel after 1 hour, add water to the lower layer of precipitate to 630ml before completely dissolving, take about 10ml of dilution, and then normalize by HPLC Detection, its total component content is 77.36%;
[0035] For the remaining 620ml, 240ml of isopropanol was added while stirring, and the layers were allowed to stand. After 1 hour, the supernatant was separated from the lower precipitate with a separatory funnel, and the lower precipitate was vacuum-dried at 80°C for 4 hours to obtain colistin sulfate dry powder 88.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com