Method for preparing monohydrate sodium carbonate and ammonium sulfate from wastes produced in process for producing sodium cyanate by urea method
A technology of sodium cyanate and sodium carbonate, applied in chemical instruments and methods, ammonium sulfate, alkali metal carbonate, etc., can solve problems such as loss, and achieve the effect of increasing economic benefits and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A method for preparing sodium carbonate monohydrate and ammonium sulfate from the waste obtained by producing sodium cyanate with the urea process, comprising the steps:
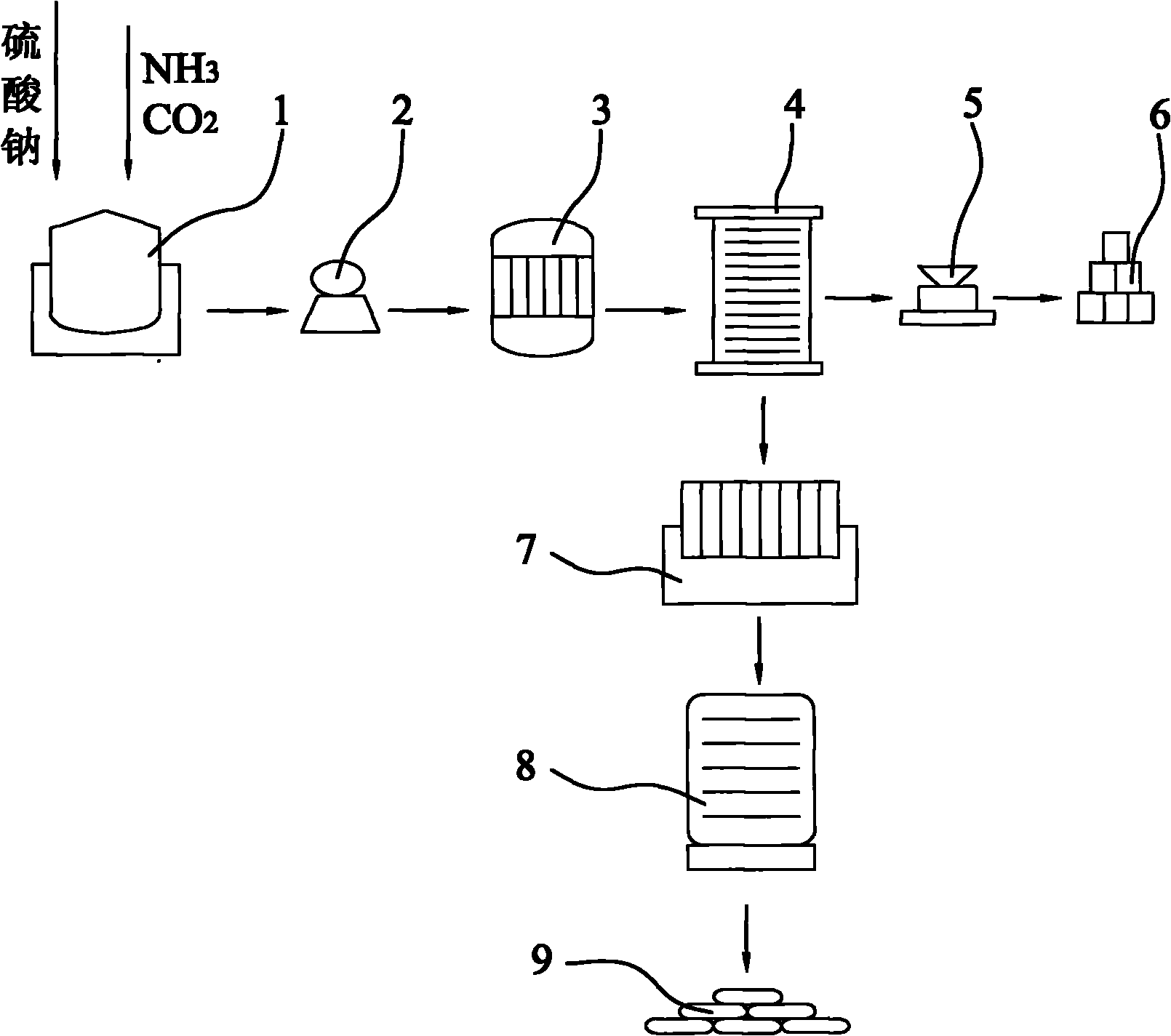
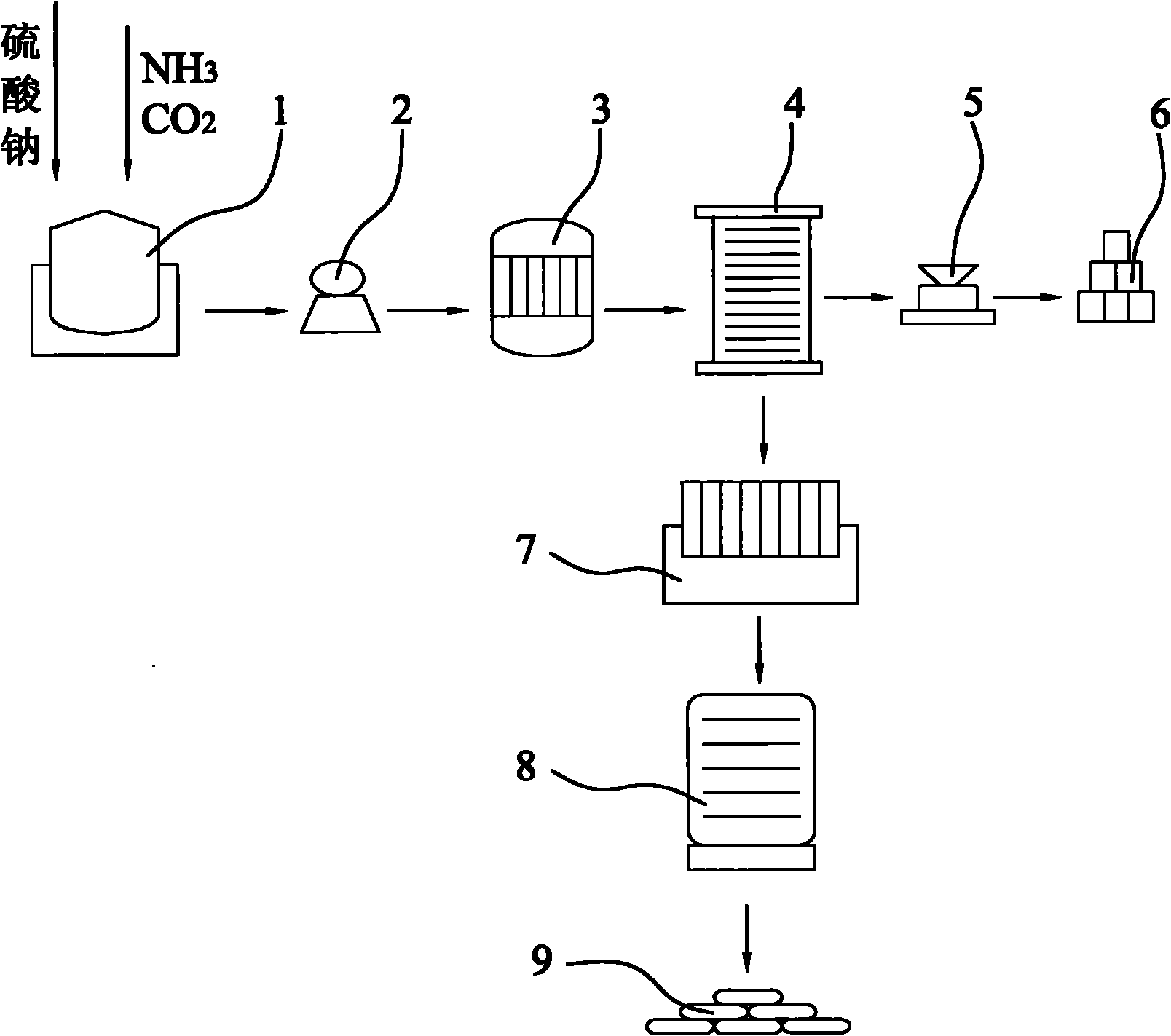
[0035] A: Send 500kg of industrial sodium sulfate to the carbonization tower (1), and feed ammonia and carbon dioxide into the carbonization tower (1) according to the mass ratio of sodium sulfate, ammonia, and carbon dioxide of 1:0.04:0.11 for carbonization reaction;
[0036] B: the reaction product obtained in step A is filtered through a suction filter pump (2) to obtain a clear filtrate and a filter cake of black impurities;
[0037]C: the clarified filtrate of step B gained is sent in the decompression distiller (3), carries out fractional distillation according to the freezing point of sodium carbonate and ammonium sulfate and cools in cooling tower (4), thereby obtains sodium carbonate decahydrate crystal respectively and ammonium sulfate crystals;
[0038] D: the ammonium sulfate crystal obt...
Embodiment 2
[0041] A method for preparing sodium carbonate monohydrate and ammonium sulfate from the waste obtained by producing sodium cyanate with the urea process, comprising the steps:
[0042] A: Send 200kg of industrial sodium sulfate to the carbonization tower (1), and feed ammonia and carbon dioxide into the carbonization tower (1) according to the mass ratio of sodium sulfate, ammonia, and carbon dioxide at 1:0.24:0.31 for carbonization reaction;
[0043] B: the reaction product obtained in step A is filtered through a suction filter pump (2) to obtain a clear filtrate and a filter cake of black impurities;
[0044] C: the clarified filtrate of step B gained is sent in the decompression distiller (3), carries out fractional distillation according to the freezing point of sodium carbonate and ammonium sulfate and cools in cooling tower (4), thereby obtains sodium carbonate decahydrate crystal respectively and ammonium sulfate crystals;
[0045] D: the ammonium sulfate crystal ob...
Embodiment 3
[0048] A method for preparing sodium carbonate monohydrate and ammonium sulfate from the waste obtained by producing sodium cyanate with the urea process, comprising the steps:
[0049] A: Send 300kg of industrial sodium sulfate to the carbonization tower (1), and feed ammonia and carbon dioxide into the carbonization tower (1) according to the mass ratio of sodium sulfate, ammonia, and carbon dioxide at 1:0.44:0.51 for carbonization reaction;
[0050] B: the reaction product obtained in step A is filtered through a suction filter pump (2) to obtain a clear filtrate and a filter cake of black impurities;
[0051] C: the clarified filtrate of step B gained is sent in the decompression distiller (3), carries out fractional distillation according to the freezing point of sodium carbonate and ammonium sulfate and cools in cooling tower (4), thereby obtains sodium carbonate decahydrate crystal respectively and ammonium sulfate crystals;
[0052] D: the ammonium sulfate crystal ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com