Application of 4-tertiary butyl-5-(1,2,4-triazole-1-base)-2-benzyliminothiazole in preparation of insecticide
An insecticide and hydroxyl technology, which is applied in the application field of 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benzyliminothiazole as the preparation of insecticides, Able to solve problems such as application without research reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
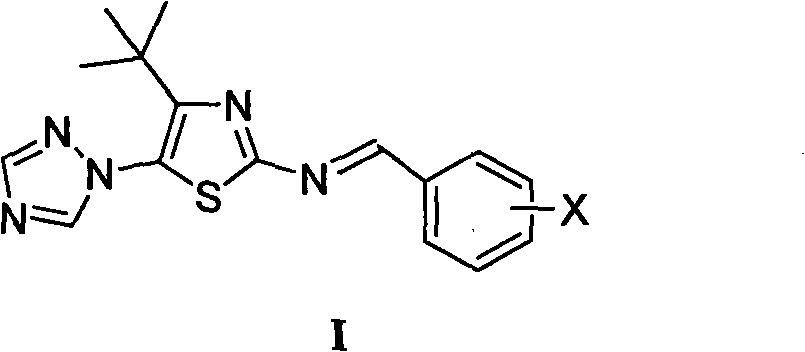
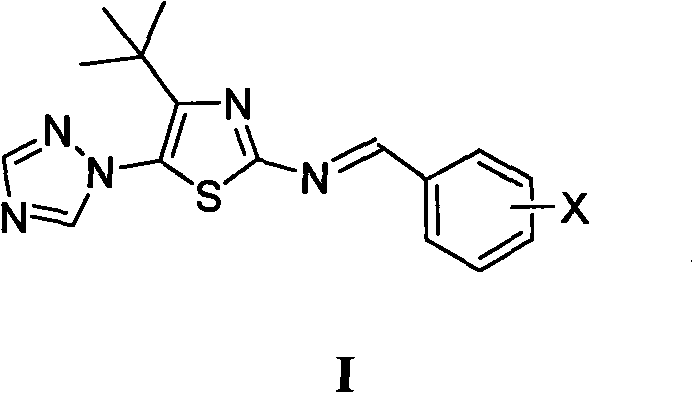
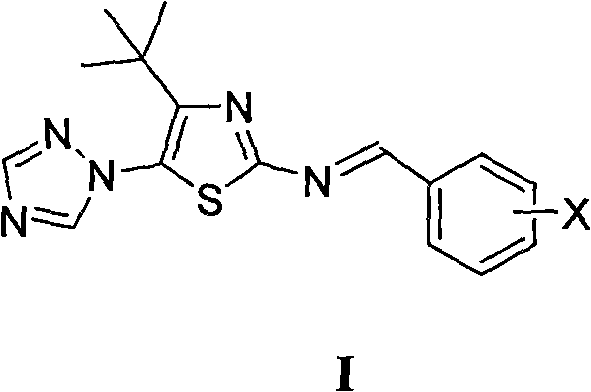
[0011] Example 1 Preparation of 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benziminothiazole (I)
[0012] Prepare 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benziminothiazole (I ):
[0013]
[0014] X is selected from: hydrogen, 2-chloro, 2-fluoro, 2-hydroxy, 2-methoxy, 2-ethoxy, 2-nitro, 3-dimethylamino, 3-chloro, 3-bromo, 3 -Fluoro, 3-methyl, 3-ethyl, 3-trifluoromethyl, 3-hydroxy, 3-methoxy, 3-ethoxy, 3-nitro, 3-sulfonic acid, 3-methyl Sulfonylamino, 3-sulfamoyl, 4-dimethylamino, 4-chloro, 4-bromo, 4-fluoro, 4-methyl, 4-ethyl, 4-trifluoromethyl, 4-hydroxyl, 4 -methoxy, 4-ethoxy, 4-acetoxy, 4-nitro, 4-sulfonate, 4-methanesulfonylamino, 4-sulfamoyl, 2-chloro-5-nitro, 3-Ethyl-4-hydroxy, 3,4-dimethoxy, 2,4-dichloro, 2-hydroxy-5-bromo, 2-hydroxy-5-iodo, 2-hydroxy-5-chloro, 2 , 4-difluoro, 2-hydroxy-3,5 dibromo, 2-hydroxy-3,5 diiodo, 2-hydroxy-3,5 dichloro or 2-hydroxy-3,5 difluoro.
Embodiment 2
[0015] Example 2 Determination of the insecticidal activity of 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benziminothiazole
[0016] 1. Purpose of the test: To determine the toxicity of 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benziminothiazole to Vicia faba aphid at the test concentration in the room Evaluation of its insecticidal activity.
[0017] 2 test conditions
[0018] 2.1 Test target: broad bean aphid (Aphis fabae) is a sensitive strain that has been raised indoors with broad bean seedlings for many years, and the test insects are 3-day-old nymphs.
[0019] 2.2 Culture conditions: The culture conditions of the test target and the test target are temperature 25±5°C, relative humidity 65±5%, and light cycle 12 / 12h (L / D).
[0020] 2.3 Instruments and equipment: electronic balance (sensitivity 1 / 10,000), 100ml beaker, measuring cylinder, petri dish, paramembrane, sponge, filter paper, pipette, tweezers, brush, etc.
[0021] 3 Experimental design
[0022] 3.1 Preferred compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com