Method for preparing rare earth element sulfide
A technology of rare earth elements and sulfides, applied in the fields of rare earth metal compounds, chemical instruments and methods, inorganic chemistry, etc., can solve problems such as darkening, dark product body color, high impurity C content, etc. Short and low reaction temperature effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
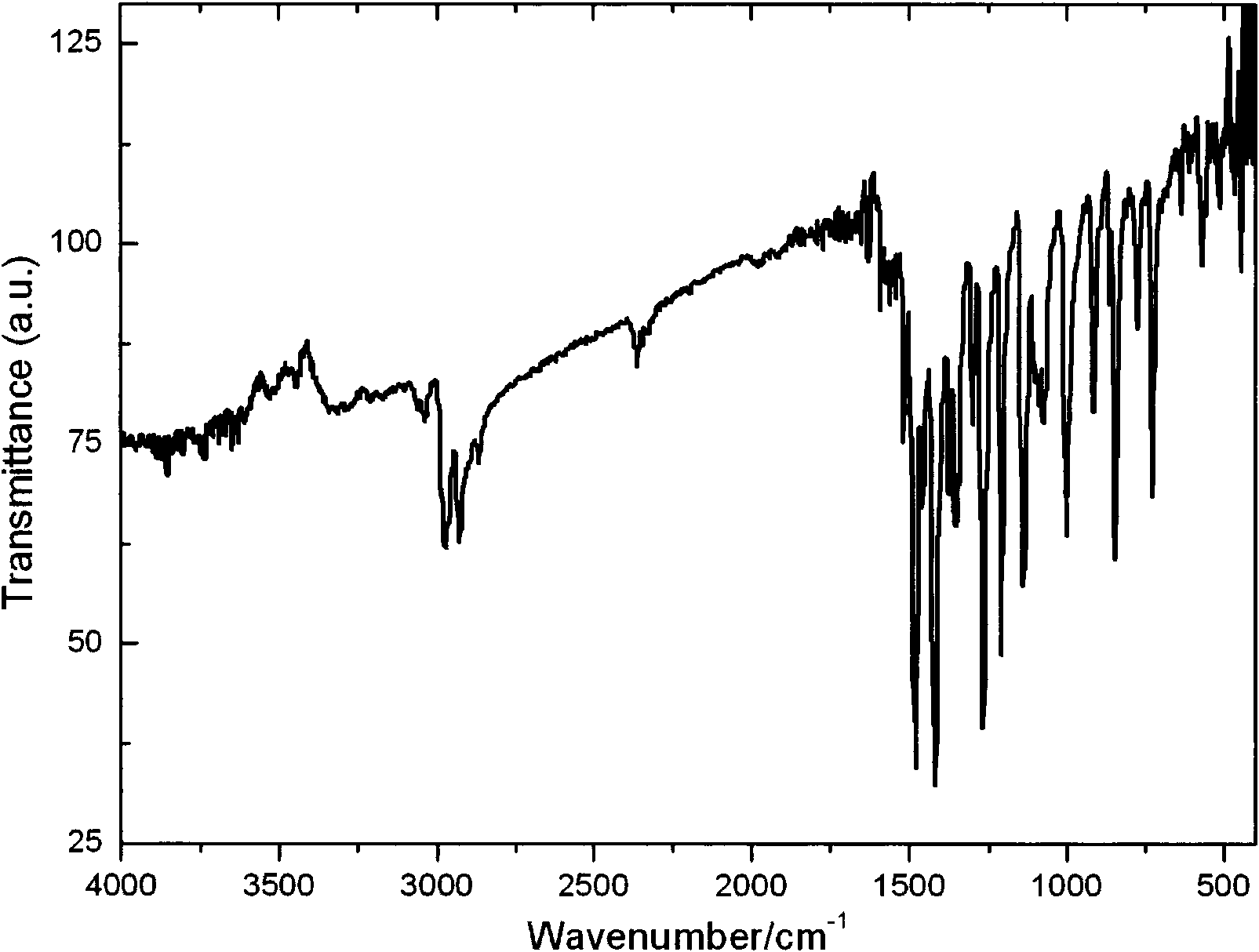
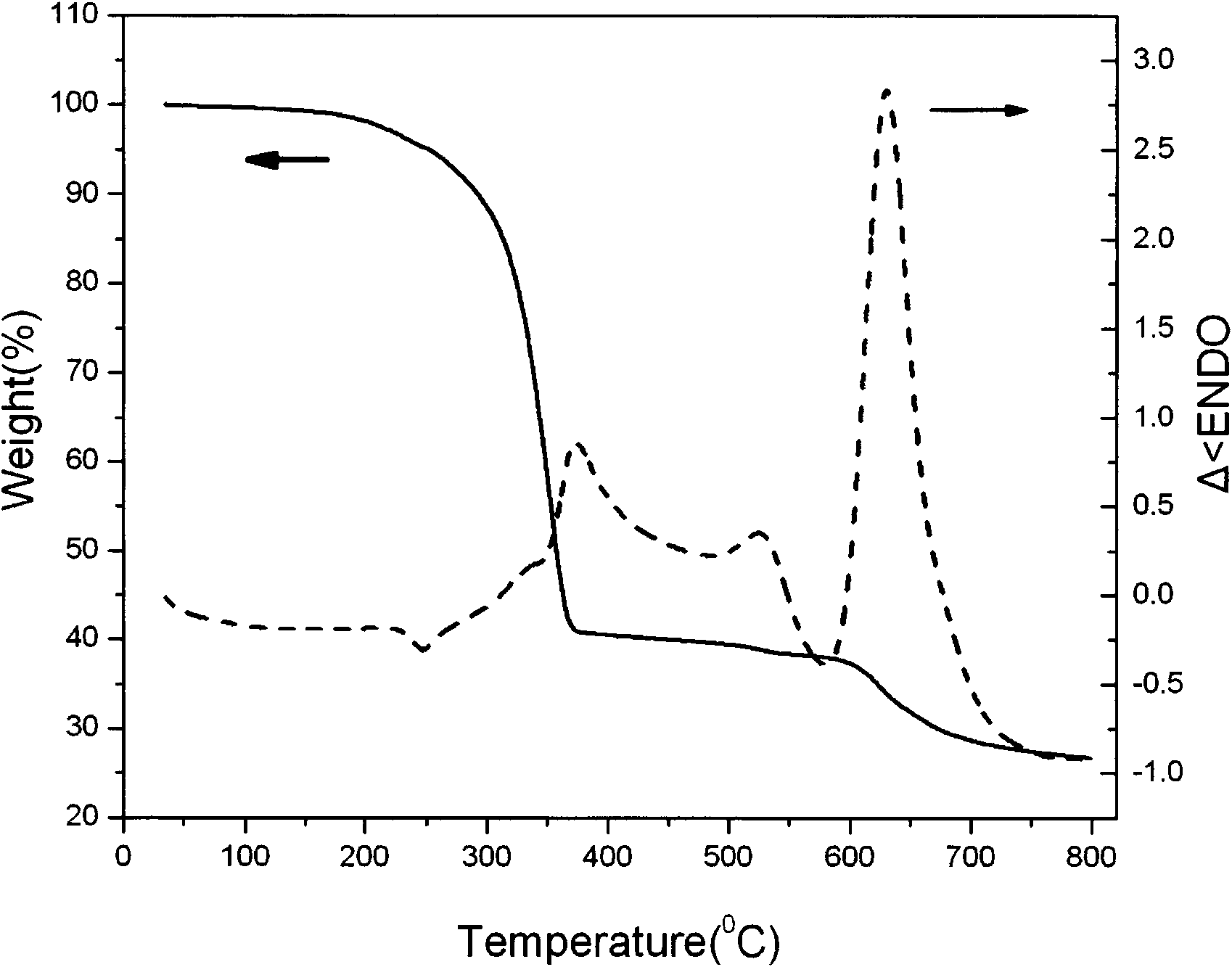
[0062] Gd(NO 3 ) 3 ·H 2 O (4.5125g, 10mmol), o-phenanthroline (phen, 1.97g, 10mmol), NaS 2 CN(C 2 h 5 ) 2 ·3H 2 O (6.76g, 30mmol) was dissolved in 50ml, 50ml, and 150ml of acetonitrile respectively, and was dissolved completely by ultrasonication. Gd(NO 3 ) 3 ·H 2 O in acetonitrile and NaS 2 CN(C 2 h 5 ) 2 ·3H 2 O was mixed with acetonitrile solution, and the resulting precipitate was removed by rapid filtration. Add the acetonitrile solution of triethyl orthoformate (3.3g) and o-phenanthroline to the filtrate, let it stand for about 10min, after the precipitate is precipitated, filter it under reduced pressure, drain it, wash it twice with acetonitrile, and dissolve the solid in 20ml of chloroform, filter, get the filtrate, and naturally evaporate the solvent at room temperature to obtain the product, place it in a desiccator, and then obtain the dry precursor (attached figure 1 , 2 ). Then add the de-C agent S to the dried precursor product, the amount of t...
Embodiment 2
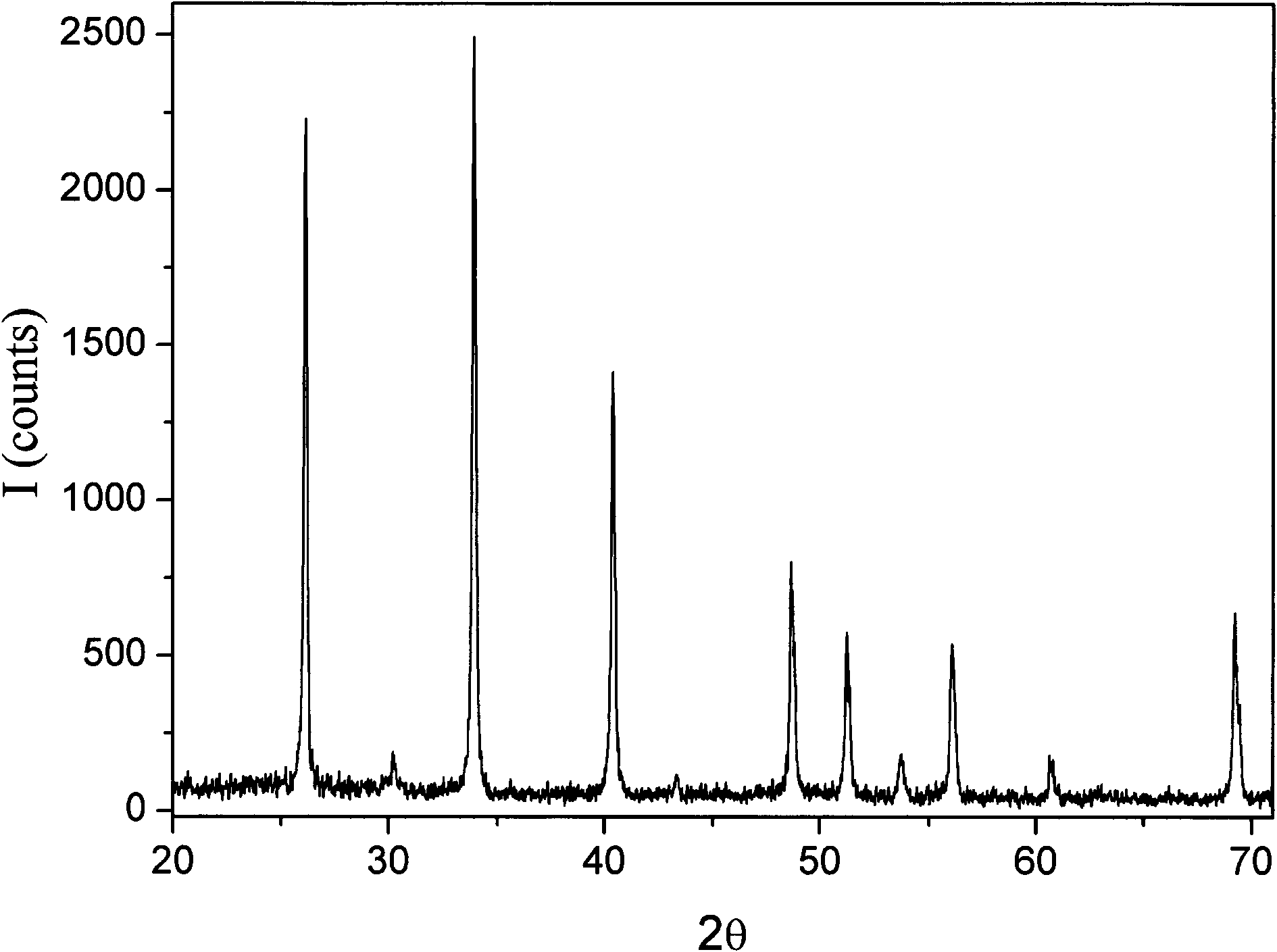
[0069] Gd(NO 3 ) 3 ·H 2 O (1.6g, 3mmol), o-phenanthroline (0.6g, 3mmol), NaS 2 CN(C 2 h 5 ) 2 ·3H 2 O (4.06g, 18mmol) was dissolved in 15ml, 15ml, and 90ml of acetonitrile respectively, and was dissolved completely by ultrasonication. Gd(NO 3 ) 3 ·H 2 O in acetonitrile and NaS 2 CN(C 2 h 5 ) 2 ·3H 2 O and acetonitrile were mixed, and the resulting precipitate was removed by rapid filtration. Add the acetonitrile solution of triethyl orthoformate (3.3g) and o-phenanthroline to the filtrate, let it stand for about 10min, after the precipitate is precipitated, filter it under reduced pressure, drain it, and place it in a vacuum desiccator to obtain dry and dry of the mixed precursor and then dry the precursor product in S v / Ar atmosphere at 1000 ° C for 240 min to obtain the composite sulfide NaGdS 2 Sample (attached Figure 4 ). In order to improve the crystallinity of the product, an appropriate amount of flux can also be added, and the flux is A 2 CO 3 、A...
Embodiment 3
[0071] Gd(NO 3 ) 3 ·H 2 O (4.5125g, 10mmol), o-phenanthroline (phen, 1.97g, 10mmol), NaS 2 CN(C 2 h 5 ) 2 ·3H 2 O (6.76g, 30mmol) was dissolved in 50ml, 50ml, and 150ml of acetonitrile respectively, and was dissolved completely by ultrasonication. Gd(NO 3 ) 3 ·H2 O in acetonitrile and tri-NaS 2 CN(C 2 h 5 ) 2 ·3H 2 O and acetonitrile were mixed, and the resulting precipitate was removed by rapid filtration. Add the acetonitrile solution of triethyl orthoformate (3.3g) and o-phenanthroline to the filtrate, let it stand for about 10min, after the precipitate is precipitated, filter it under reduced pressure, drain it, wash it twice with acetonitrile, and dissolve the solid in 20ml of chloroform, filter, take the filtrate, and naturally evaporate the solvent at room temperature to obtain the product, and place it in a desiccator to obtain a dry precursor. Then the dried precursor product was mixed with an equimolar ratio of Zn(S 2 CN(C 2 h 5 ) 2 ) 2 Mixed, in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com