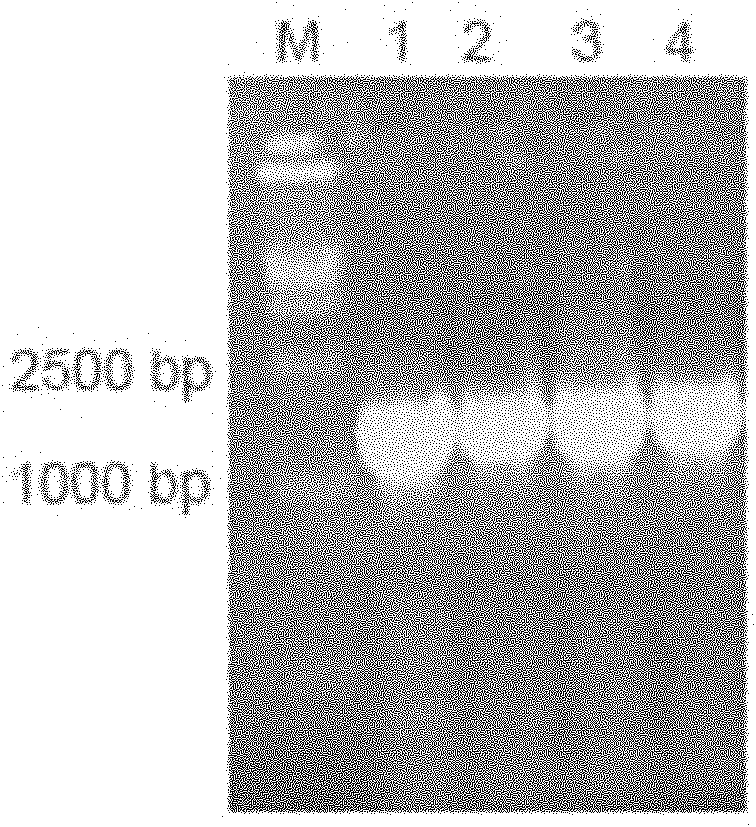Interferon fusion protein, preparation thereof and application thereof
A fusion protein, interferon technology, applied in the direction of peptide/protein components, hybrid peptides, drug combinations, etc., can solve the problems of long half-life therapeutic effect, short half-life, low interferon activity, etc. High and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
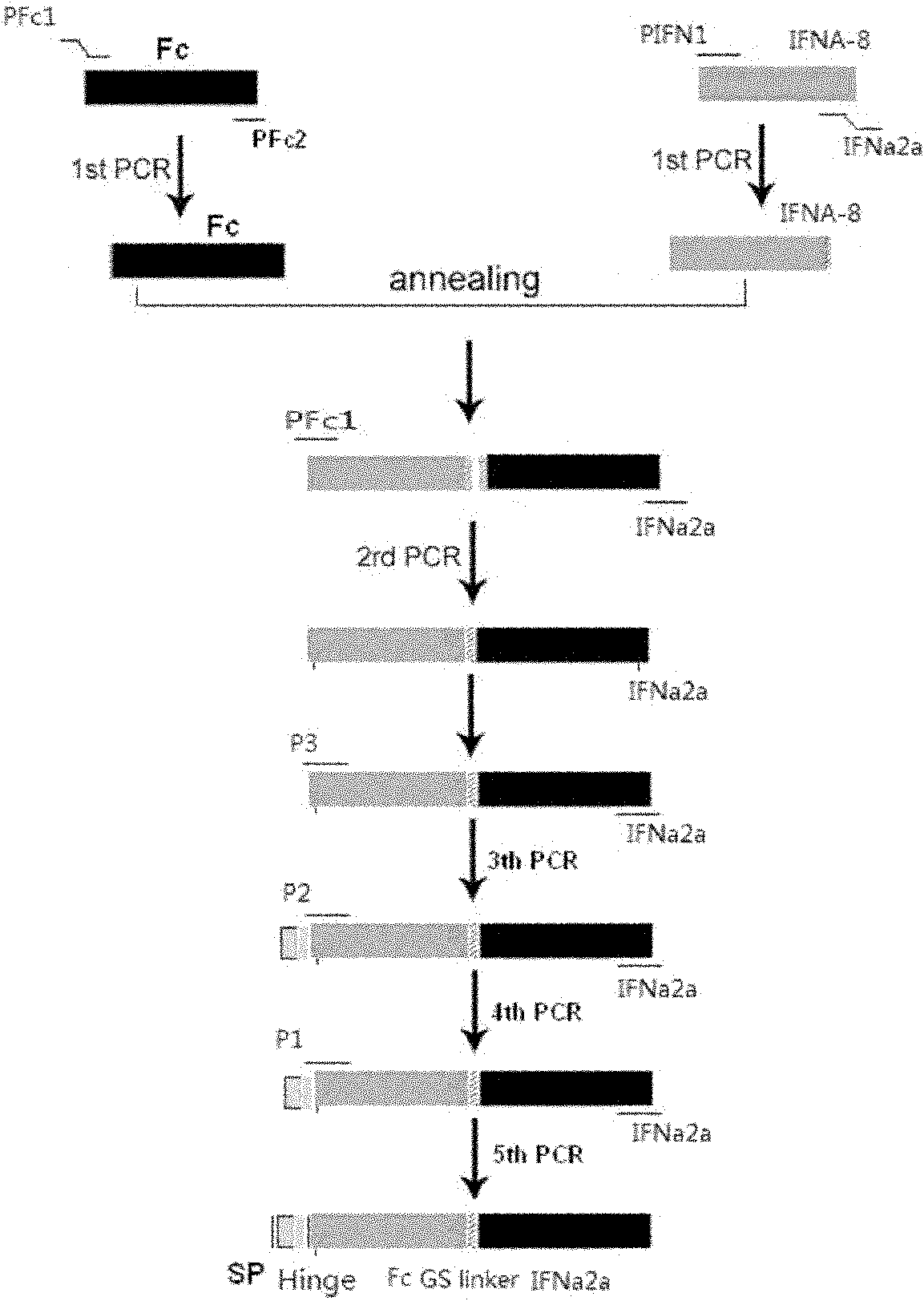
[0059] Example 1: Obtaining IFNɑ2a target gene and human IgG2 Fc (hinge region+CH2+CH3) fragment gene
[0060] According to the IFNɑ2a gene sequence (NM_000605), it was sent to Gene Synthesis Company for synthesis. Using the total RNA of normal human lymphocytes as a template, the primers are PFc1: 5' GAC AAG ACC CAC ACC TGTC 3', PFc2: 5' GCCGCCAGATCCGCCTCCGCCCTTTCCGGGGCTCAG 3', RT-PCR amplifies the IgG2 Fc fragment, the reaction process is as follows, reverse transcription reaction : 70°C for 10 minutes, 42°C for 30 seconds, 94°C for 5 minutes and 4°C for cooling. PCR reaction: Denaturation at 94°C for 30 seconds; annealing at 55°C for 30 seconds; extension at 72°C for 1 minute, 30 cycles of reaction. This was followed by an additional extension at 72°C for 5 minutes. After the reaction was completed, RT-PCR products were detected by 1% agarose gel electrophoresis. The flow chart of the whole gene splicing is as follows: figure 1 as shown, figure 2 Indicates that we hav...
Embodiment 2
[0061] Embodiment 2: Construction of recombinant plasmid
[0062] 1) Overlap extension splicing technique (over-lap) to connect IFNɑ2a and Fc genes: Primers were designed as follows: The following primers were designed according to IFNɑ2a and human IgG2 Fc (hinge region+ CH2+ CH3) cDNA sequences:
[0063] PFc1: 5’ GAC AAG ACC CAC ACC TGT C 3’
[0064] PFc2: 5’ GCCGCCAGATCCGCCTCCGCCCTTTCCGGGGCTCAG 3’
[0065]PIFN1: 5'TCTGGCGGCGGAGGAAGTGGCGGAGGCTCTGGCTGCGACCTGCCTCAG 3'
[0066] PIFN2: 5’ CGCGGATCCTCA CTC TTT GGA CTT CAG 3’
[0067] PIFN2 contains a BamH1 restriction site. PFc2 and PIFN1 contain a glycine-serine linking peptide (Gly3Ser) sequence
[0068] 2) PCR reaction system
[0069] Set up the following reaction system in a 50 μl PCR reaction tube
[0070] 10×PCR buffer
5 μl
cDNA
2 μl (10 ng)
PFc1 (10 mM)
2 μl
PFc2: (10 mM)
2 μl
dNTP
4 μl
Taq enzyme
0.5μl (2.5U)
Mg ions
3 μl
[0071] ...
Embodiment 3
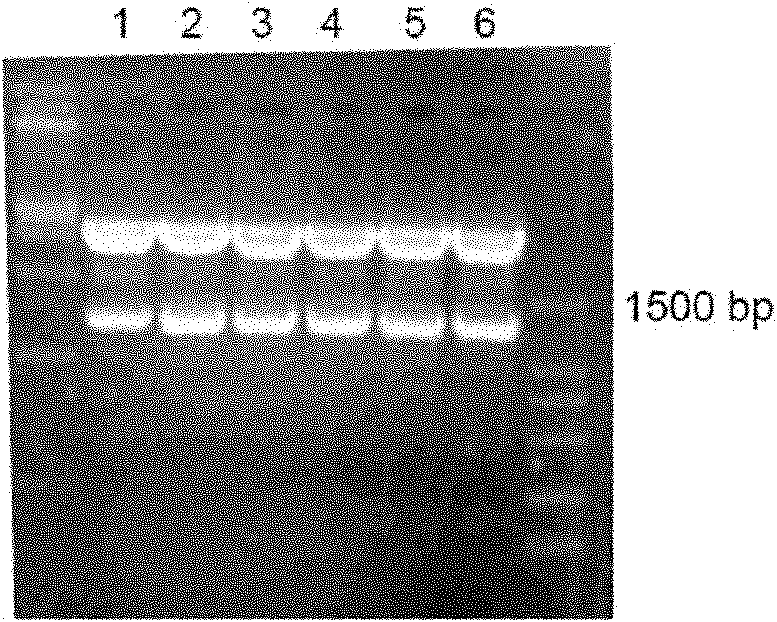
[0159] Example 3: Screening and Identification of CHO Stable Expression Strains
[0160] 1) Take 10ug of the pFc-IFNɑ2a plasmid prepared in large quantities in Example 2, and use liposome Lipofectin2000 to transfect CHO cells. Two days later, passaging at a ratio of 1:5, adding 0.4mg / ml G418 for selection, clonal formation can be seen in 10 days. Random digestion of 168 monoclonals with distinct borders and good cell status was inoculated into seven 24-well plate cultures (the first round of screening).
[0161] 2) Use ELISA to detect the expression of the fusion protein on the three-day culture, and select 45 positive clones to inoculate two 24-well plates (the second round of screening) and one 6-well plate (for After 4 days of culture, the supernatant was taken to detect the expression of the fusion protein by ELISA, and 6 clones with higher expression were selected: A2-6, B1-5, C6-3, D5-5, D6-4, D6-5 was further screened by limiting dilution method.
[0162] 3) Inocula...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com