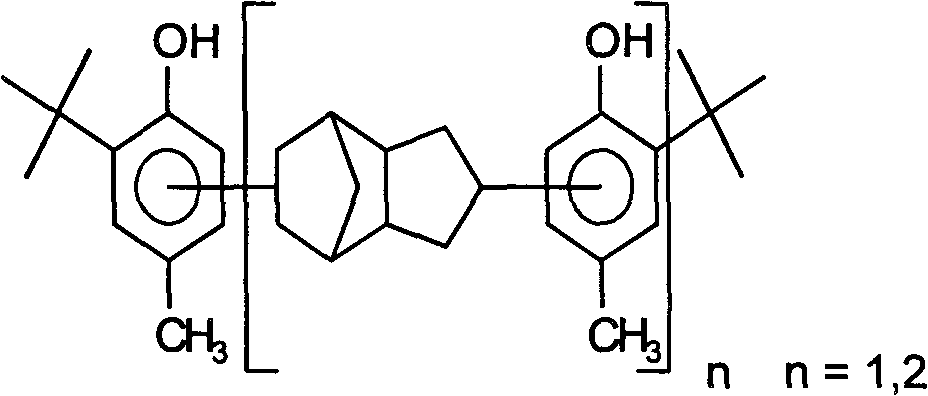
Synthesis method of paracresol-dicyclopentadiene isobutylation resin antioxidant
A technology of dicyclopentadiene isobutyl and p-cresol, which is applied in the synthesis of hindered phenol antioxidants, can solve the problems of difficult removal of catalyst, poor product quality, and poor product chromaticity, and increase the utilization rate of equipment , low ash content and reduced equipment investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In a reactor equipped with a stirrer, a reflux condenser, a feeding device and a 250ml nitrogen protection device, add 50g of p-cresol (purity 99.5%), 2g of boron trifluoride complex catalyst, and heat to 60-80 °C, after p-cresol is completely melted, start stirring and raise the temperature to 90-100 °C. At the same time, prepare 30g of dicyclopentadiene, slowly add it into the reactor through the feeding device, after all the dicyclopentadiene is added dropwise, keep the reaction mixture for about 4 hours, control the temperature at 100-105°C, and then lower the temperature to 100°C Below ℃, recover unreacted p-cresol (3.04Kpa, 180℃) under negative pressure, then add toluene and organic composite sulfonic acid catalyst 1.5g to carry out alkylation reaction, feed isobutylene at 75℃ until no absorption stops, add soda ash 1.5g was neutralized, the toluene was recovered by distillation, and the negative pressure (3.0Kpa) was distilled to 240°C to obtain 58g of the produc...
Embodiment 2
[0022] 800 grams of solid p-cresol was charged into a 3000 ml glass kettle, and the air was replaced with nitrogen. Heat up to melting, turn on the stirring blade (rotating speed 60-80 rpm), add 20 grams of condensation catalyst boron trifluoride complex with a dropping funnel, and continue to stir and heat up to 100°C, start adding dicyclopentadiene dropwise 450g. The reaction temperature was controlled at 100±2°C, and the stirring speed was slightly faster during the dropwise addition. After the dropwise addition, keep warm at 100±2°C for 1 hour. Under vacuum (3.04Kpa, 180°C), the moisture, excess p-cresol and small molecular substances were evaporated, and the sampling analysis was completed. Lower the material temperature below 100°C, add toluene (or benzene, or xylene), 40g of organic composite sulfonic acid catalyst and stir, and when the temperature drops to 80±2°C, feed isobutylene to control the reaction temperature. Stop when isobutene is no longer absorbed and in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com