Method for purifying and preparing allergen vaccine
A purification method and allergen technology, which is applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, antibody medical ingredients, etc., can solve the problems of many impurities and achieve the effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
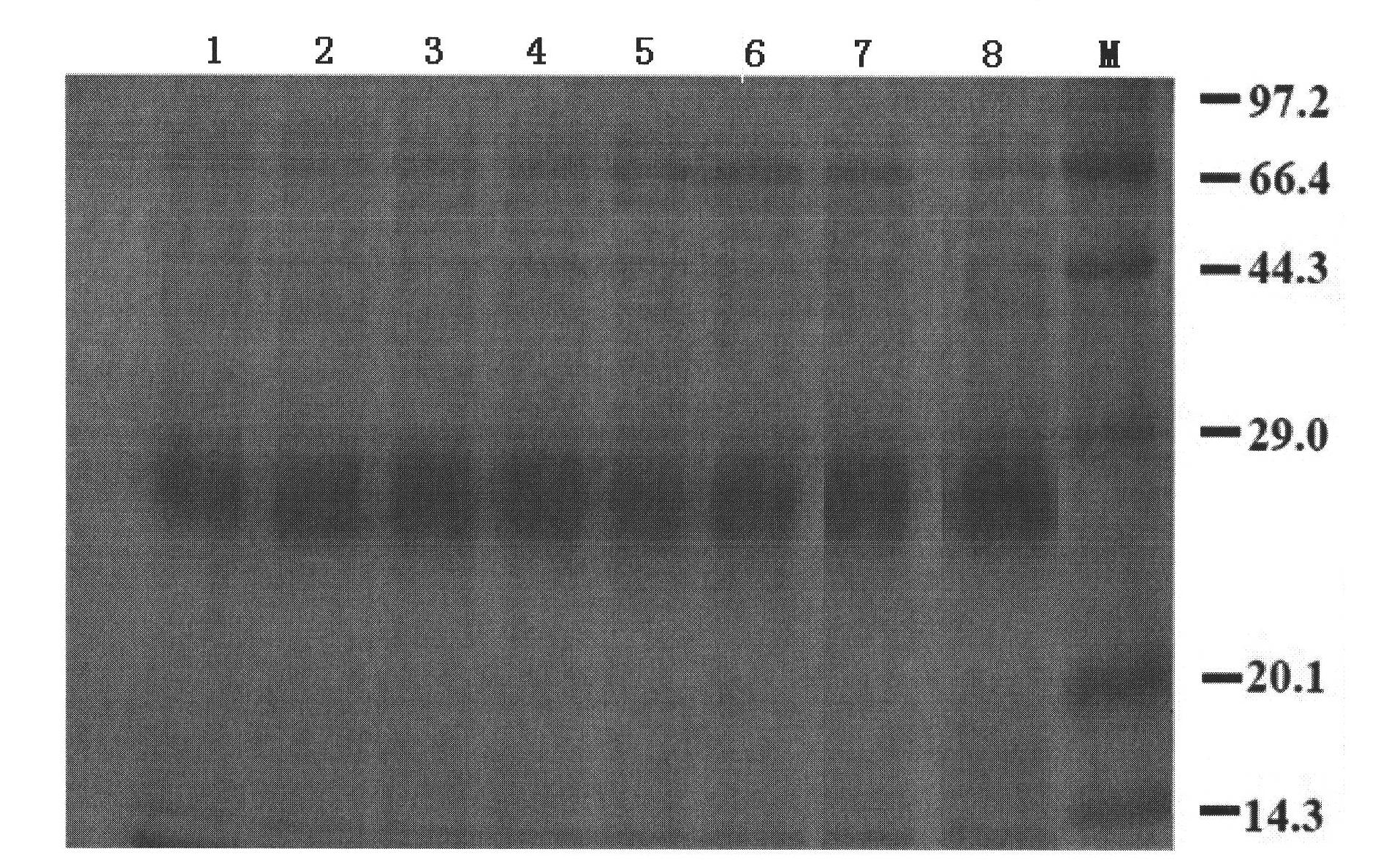
Examples
Embodiment 1
[0051] Example 1 Preparation of Artemisia annua Pollen Allergen Vaccine
[0052] Take the qualified Artemisia annua pollen, add acetone at 1:2 (volume ratio) and repeatedly degrease intermittently, until the supernatant solvent that has been left standing is colorless, pour off the solvent, and dry the residue in a fume hood. After degreasing, the pollen cannot remain. solvent. Weigh defatted pollen, add buffer (PH: 8.0200mmolNa 2 HPO 4 NaH 2 PO 4 50mmol NaCl 0.4% phenol), stirred on a magnetic stirrer at 4°C for 24h, and transferred the mixture to a 500ml centrifuge bottle. Centrifuge at 6000rpm at 4°C for 15min, take the supernatant and discard the residue, centrifuge at 8000rpm at 4°C for 30min, take the supernatant and discard the residue, and filter the supernatant with ordinary filter paper for clarification. The clarified extract is ultrafiltered through a 100KD membrane, the filtrate is collected, and the filtrate is then ultrafiltered through a 5KD membrane, an...
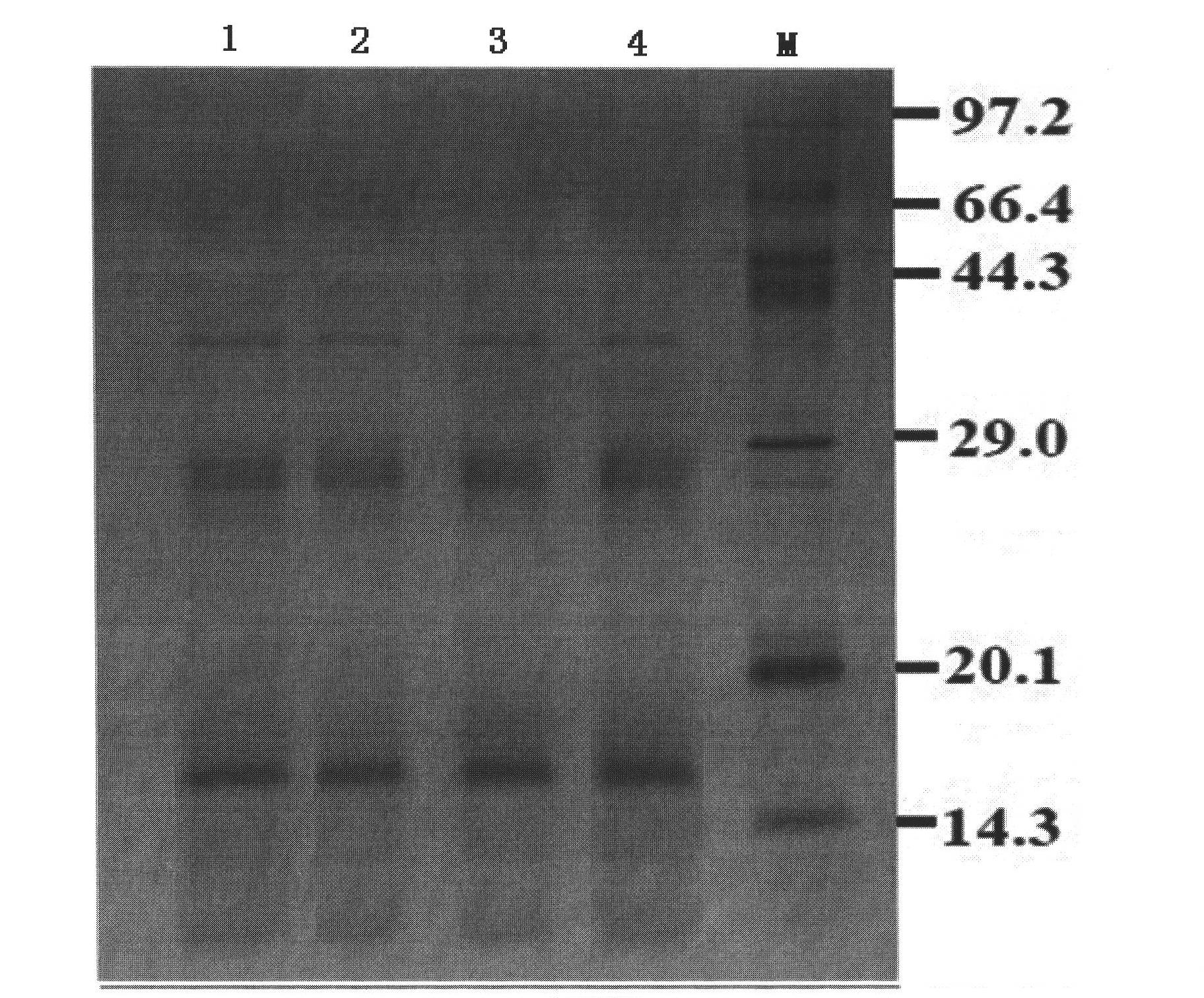
Embodiment 2
[0053] Preparation of Example 2 Household Dust Mite Allergen Vaccine
[0054] The pure mite body of dust mite that has passed the inspection is degreased by adding acetone in a ratio of 1:2 and stirring intermittently until the supernatant solvent is colorless, pour off the degreasing solvent, and dry the residue in a fume hood to keep no acetone remaining in the degreasing raw material. Weigh the degreased raw material, add 50mmol NH at a ratio of 1:25 4 HCO 3 , stirred on a magnetic stirrer at 4°C for 24h, and transferred the mixture to a 500ml centrifuge bottle. Centrifuge at 6000 rpm at 4°C for 15 minutes, take the supernatant and discard the residue, and filter the supernatant with ordinary filter paper for clarification. The clarified extract is ultrafiltered with a 100KD membrane, the filtrate is collected, and the filtrate is then ultrafiltered through a 5KD membrane. During the ultrafiltration process, the buffer solution is continuously added until the filtrate is ...
Embodiment 3
[0055] The preparation of embodiment 3 spruce pollen allergen vaccine
[0056] The spruce pollen that passed the inspection was repeatedly degreased by adding ether in a ratio of 1:2 until the supernatant solvent that stood still was colorless, poured off the solvent, and dried the residue in a fume hood. The pollen after degreasing did not leave any degreasing solvent. Weigh defatted pollen, add buffer (PH: 8.0200mmolNa 2 HPO 4 NaH 2 PO 4 50mmol NaCl 0.4% phenol), stirred on a magnetic stirrer at 4°C for 24h, and transferred the mixture to a 500ml centrifuge bottle. Centrifuge at 6000rpm at 4°C for 15min, take the supernatant and discard the residue, centrifuge the supernatant at 8000rpm at 4°C for 30min, take the supernatant and discard the residue, filter the supernatant through ordinary filter paper, and then filter through a 0.45um membrane filter to obtain the clarified stock solution 100kKD membrane ultrafiltration, collect the filtrate, and then pass through the 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com