Recombinant production method of microbial transglutaminase
A technology of transglutaminase and production method, which is applied in the field of protein and enzyme engineering, and can solve the problems of cumbersome steps, great influence of medicinal protein modification, strong affinity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
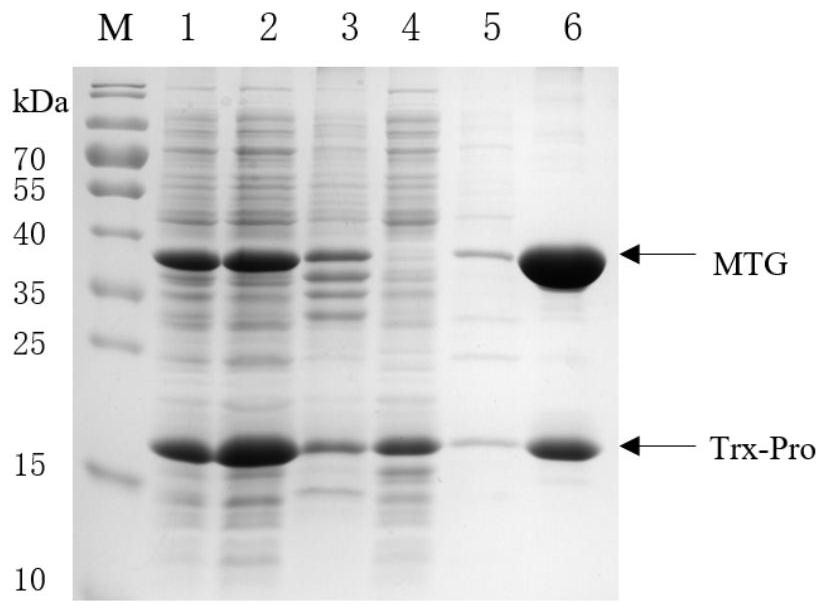
[0041] Example 1: Construction of transglutaminase recombinant expression host and protein expression and purification
[0042] (1) Construction of MTG recombinant plasmid
[0043] Connect 6 histidine purification tags to the C-terminus of mature MTG, which is convenient for the subsequent separation and purification of mature MTG. The amino acid sequence of MTG and the His6 tag are connected by G-S-L-E tetrapeptide, denoted as MEG-His6. The amino acid sequence of MEG-His6 is as follows As shown in SEQ ID NO.1, the coding gene was designed according to the above-mentioned amino acid sequence, and the nucleotide sequence of the coding gene was shown in SEQ ID NO.2. The nucleotide sequence was optimized by codons and was suitable for recombinant expression in Escherichia coli. Synthesize MTG-His6 encoding gene; when artificially synthesizing MTG-His6 encoding gene, NcoI and BglII restriction endonuclease sites were added at both ends of the nucleotide sequence;
[0044] The art...
Embodiment 2
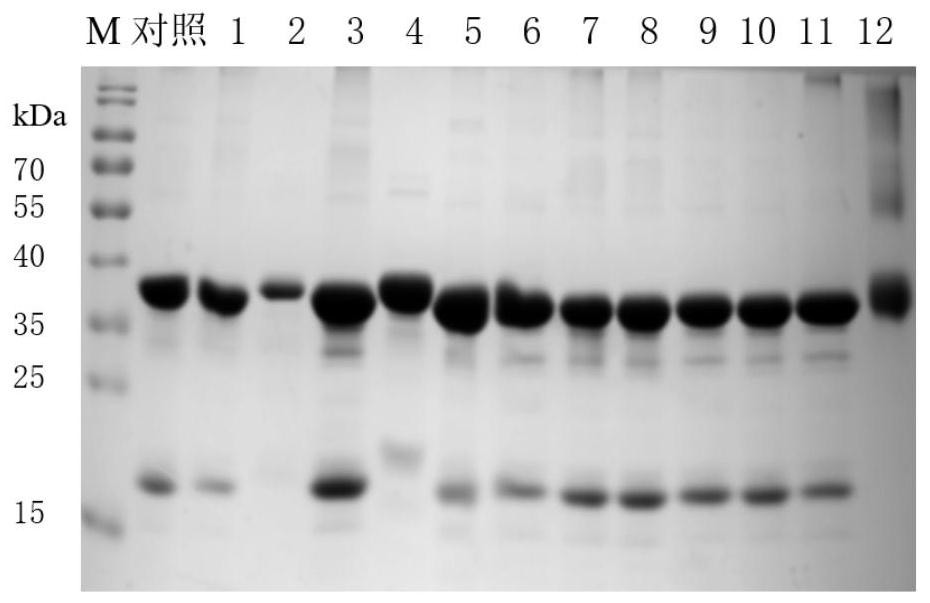
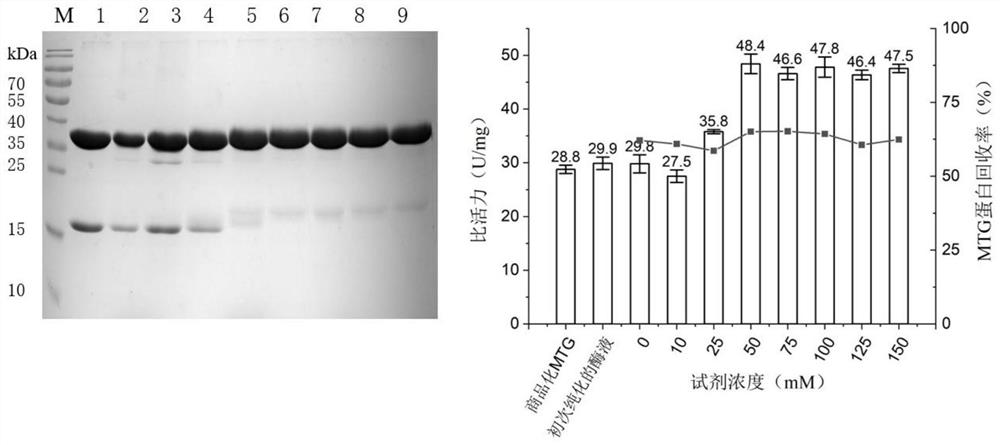
[0054] Example 2: Optimization of "molecular chaperone" Trx-Pro stripping conditions and acquisition of highly active transglutaminase
[0055] The primary purified protein obtained in Example 1 was subjected to secondary purification treatment using different treatment conditions, the protein concentration was determined using an ultra-micro spectrophotometer, and the protein composition was analyzed using SDS-PAGE. Enzyme Activity Detection Method" to measure the specific activity of transglutaminase.
[0056] (1) Screening of treatment reagents
[0057] Use dimethyl sulfoxide as a solvent to dissolve the small molecule treatment reagent, wherein the small molecule treatment reagent includes fluorescein isothiocyanate, 3-(4-hydroxyphenyl) propionate N-hydroxysuccinimide ester, 4-benzene ureaazole, acetate-N-succinimidyl ester, 4-phenyl-1,2,4-triimidazole-3,5-dione, 4-(aminomethyl)phenol, N-acetyl-L Tyrosine, glycyl-L-tyrosine hydrate, 4-hydroxybenzyl alcohol, L-tyrosine, 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com