DNA vaccine adjuvant using Micro RNA-155 and construction method thereof
A technology of nucleic acid vaccine and construction method, which is applied in the fields of medical biology and immune activity enhancement of nucleic acid vaccines, and can solve the problems of not using MicroRNA-155 plasmid carrier vaccine adjuvant research reports, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
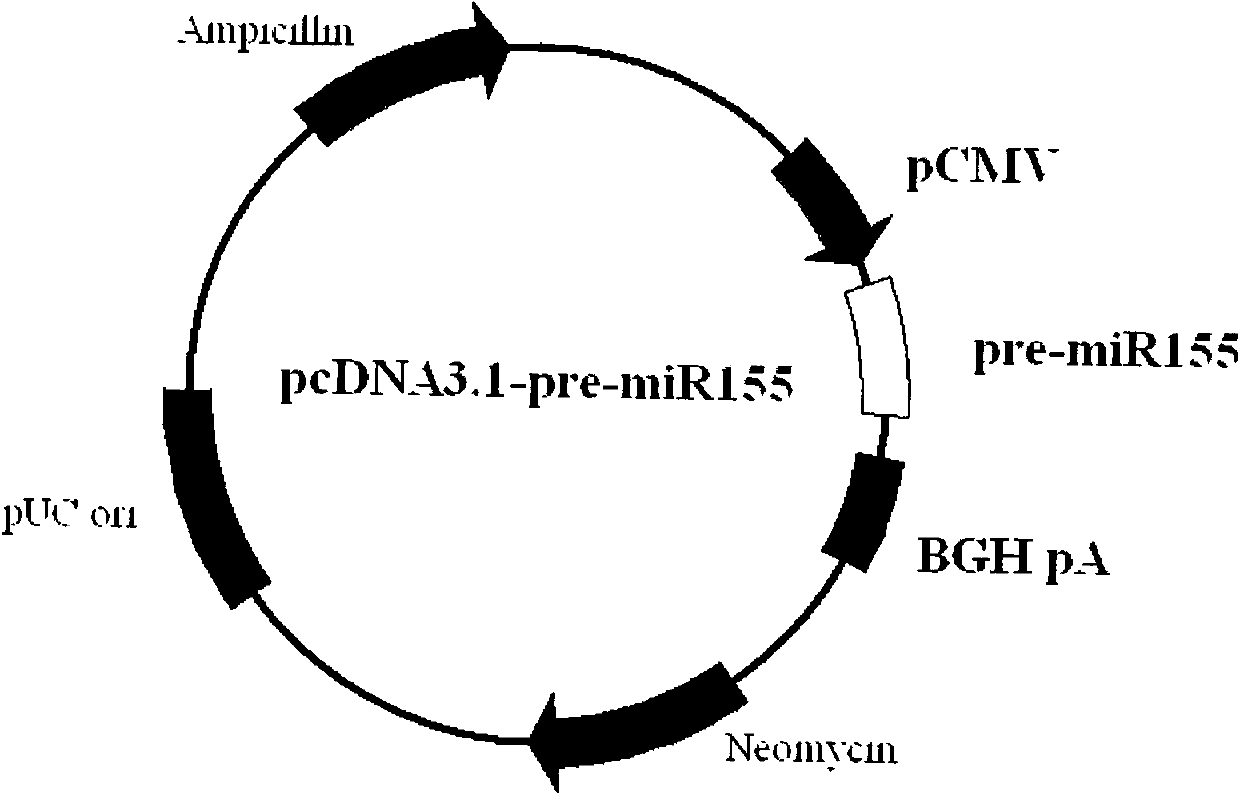
[0026] Embodiment 1: Construction of pcDNA3.1-pre-miR-155 plasmid
[0027] We first searched the genome sequence encoding the human microRNA-155 precursor molecule (GENBANK: NR_030784, shown in SEQ ID NO: 1) according to the Internet database information (http: / / www.mirbase.org), and designed PCR for its flanking sequence Primers (BamH I and EcoR I restriction sites are added to the upstream and downstream, respectively), using genomic DNA extracted from human peripheral blood mononuclear cells for PCR, the microRNA-155 precursor molecular sequence and PCR primers for cloning are shown in the table below. Thus, a DNA fragment containing the coding sequence of the microRNA-155 precursor molecule was obtained.
[0028]
[0029] The DNA fragment comprising the pre-miR-155 coding sequence of the above clone (such as figure 1 and the following sequences), using BamH I and EcoR I restriction sites. The polyclonal restriction sites flanking the insertion sequence are still retai...
Embodiment 2
[0031] Example 2: In vitro expression verification of pcDNA3.1-pre-miR-155 plasmid
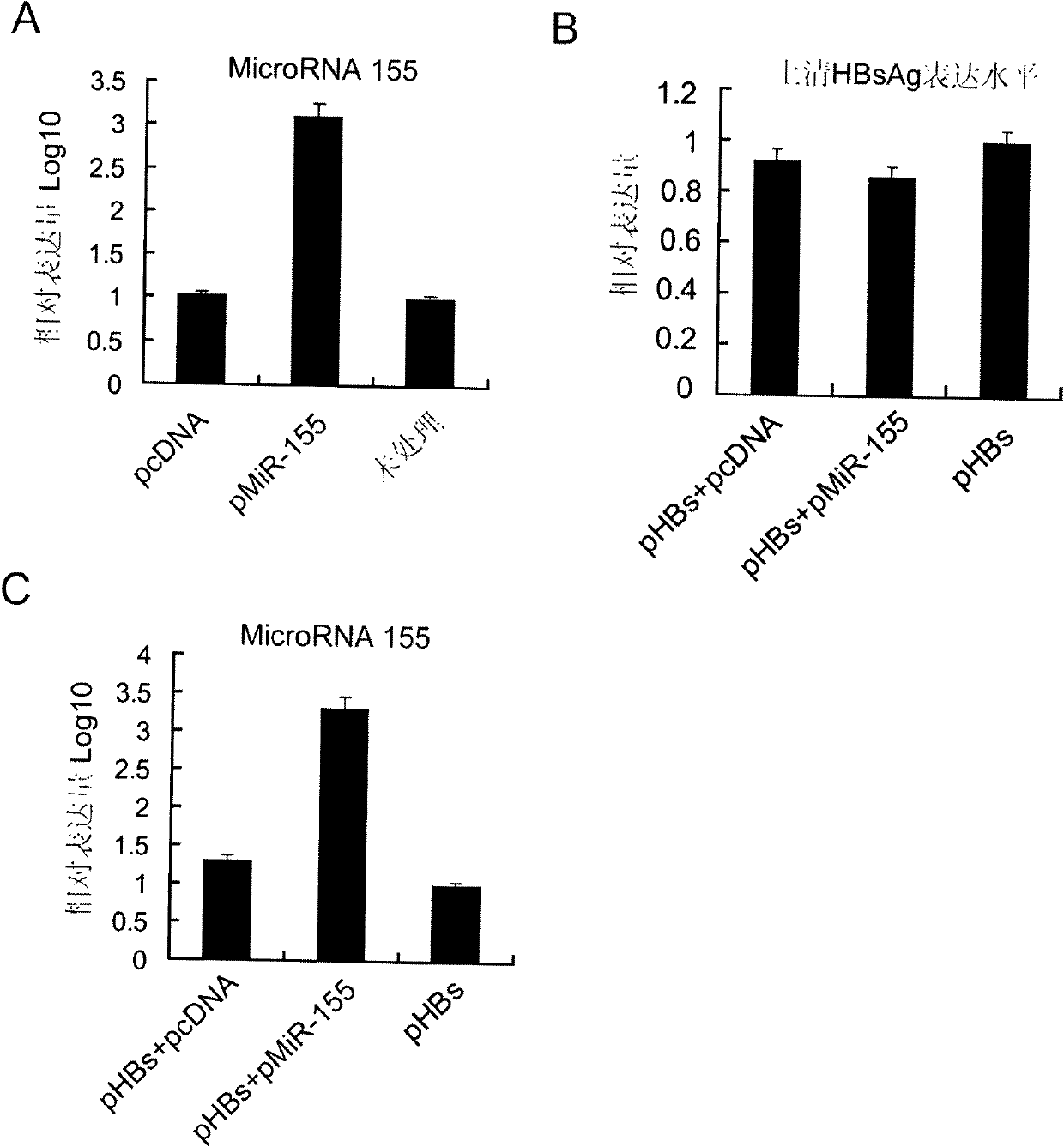
[0032] Using 293T cells (purchased from ATCC, USA) as an in vitro model, cells were transfected with pcDNA3.1-pre-miR-155 plasmid (pMiR-155) using Lipofectamine2000 transfection reagent (Invitrogene, USA, refer to its instruction manual). Set up pcDNA3.1 (pcDNA) empty plasmid transfection group and untreated group (Untreated) as controls. After 24 hours, the total cellular RNA was extracted with Trizol reagent (Invitrogene, USA, refer to its instruction manual), and the microRNA real-time quantitative detection kit of Guangzhou Funeng Gene Co., Ltd. was used to detect intracellular has -expression level of microRNA-155 mature body, it was found that the expression level of has-microRNA-155 mature body increased by more than 1000 times after transfection of pcDNA3.1-pre-miR-155 plasmid (such as figure 2 shown in A).
Embodiment 3
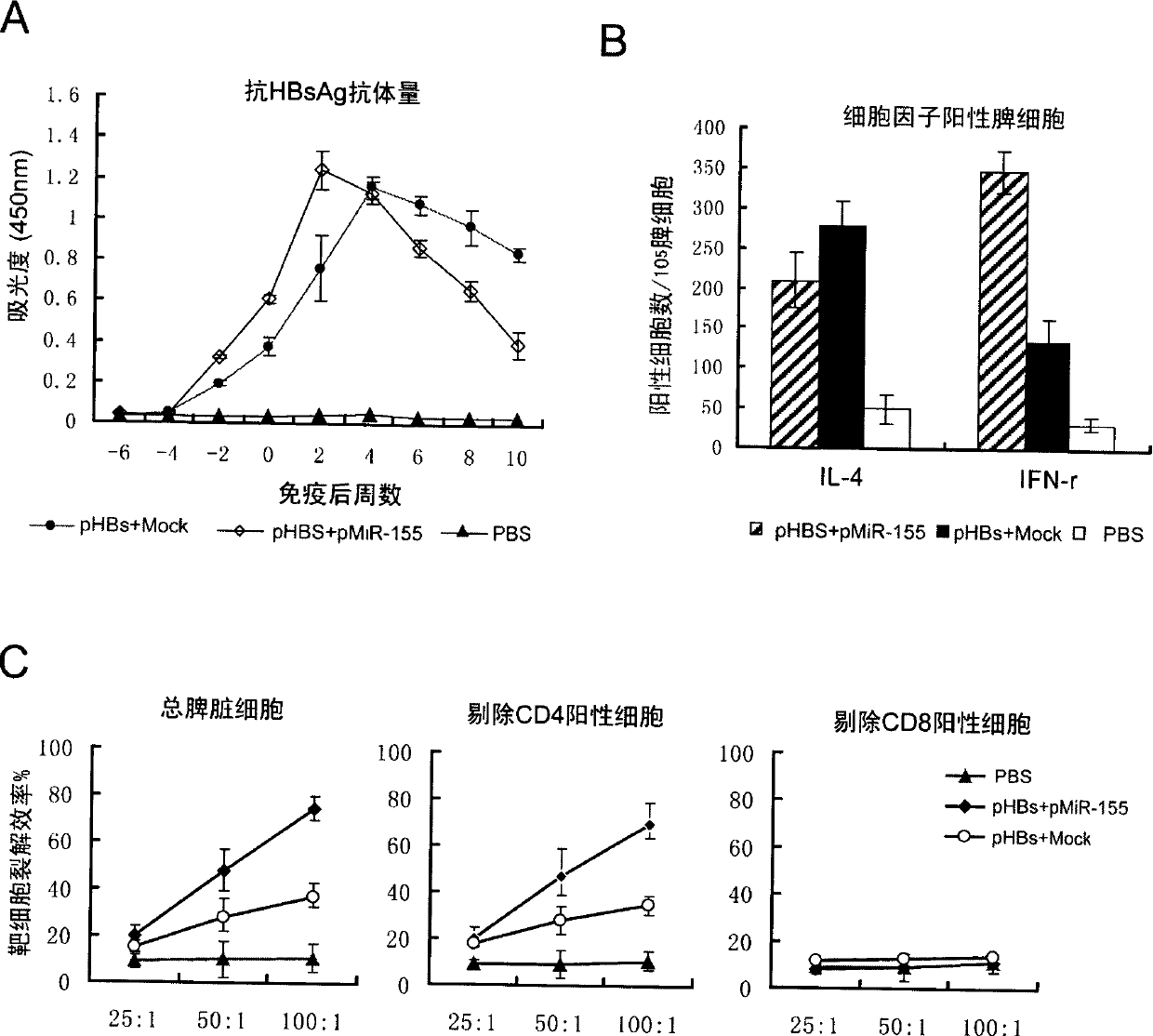
[0033] Example 3: Detection of expression ability in vitro under the condition of co-transfection of pcDNA3.1-pre-miR-155 plasmid and pVAX1-HBsAg nucleic acid vaccine.
[0034] Using 293T cells as an in vitro model, use Lipofectamine2000 transfection reagent z to transfect the following three groups of plasmids, 1. pVAX1-HBsAg and pcDNA3.1 empty plasmid group (pHBs+pcDNA) transfection; 2. pcDNA3.1-pre-miR- 155 plasmids were transfected with pVAX1-HBsAg (pHBs+pMiR-155); 3. pVAX1-HBsAg was transfected alone (pHBs). After 24 hours, the expression level of HBsAg antigen in the cell culture supernatant was detected by double antibody sandwich ELISA method, and it was found that after co-transfection of pcDNA3.1-pre-miR-155 plasmid and pVAX1-HBsAg, pVAX1-HBsAg could still express HBsAg efficiently Antigen, comparable to the expression level of pVAX1-HBsAg (eg figure 2 shown in B).
[0035] The cells treated in the above three groups were extracted with Trizol reagent from America...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com