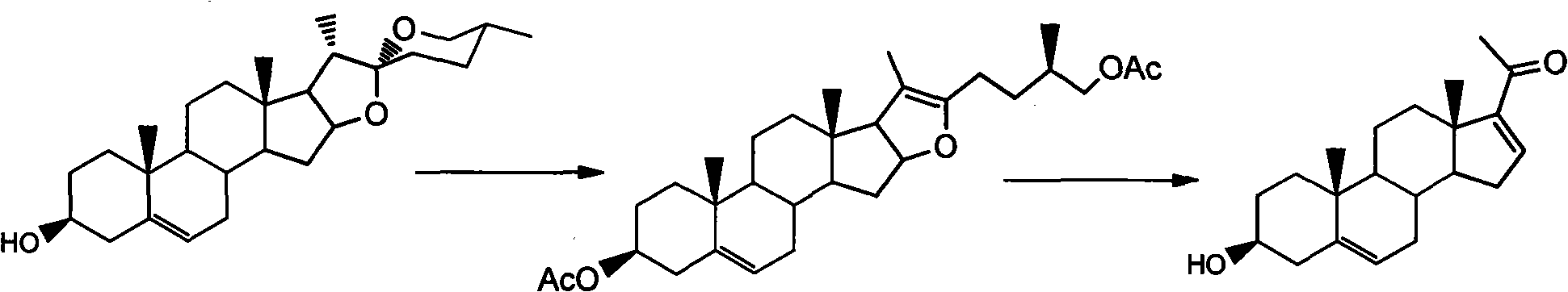
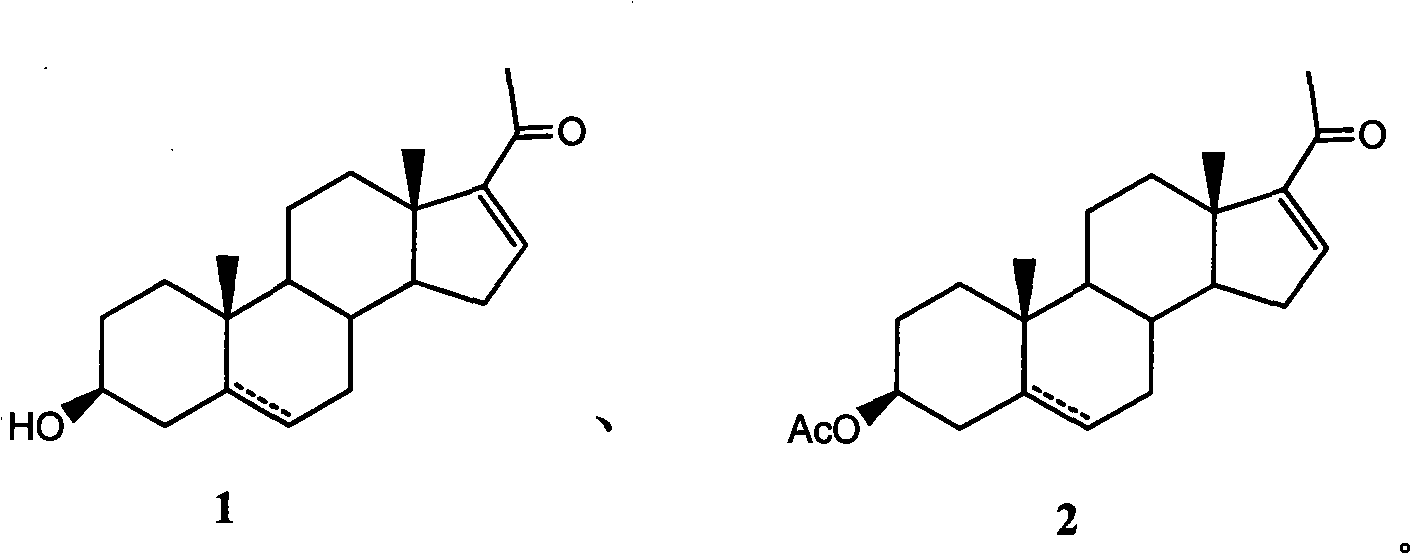
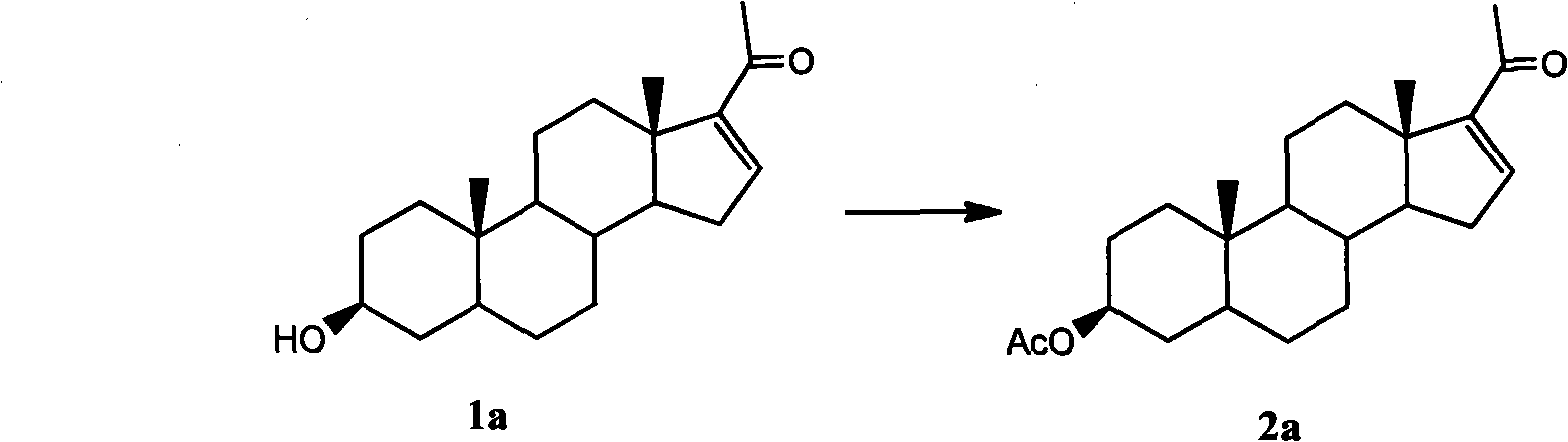
Purification technology of pregnenolone acetate
A technology for pregnenolone acetate and pregnenolone alcohol, which is applied in directions such as organic chemistry and steroids, can solve problems such as high cost, influence on the production and application of pregnenolone alcohol, and achieves the effect of easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Synthesis and purification of embodiment 1 compound 2a
[0019]
[0020] Put 50 g of pregna-16-en-20-one-3-ol crude product (content 88%) in a dry 1 liter three-neck reaction flask, add 120 ml of acetic anhydride, and heat to dissolve. After reacting at 100°C for 1 h, the acetylation reaction was complete. After cooling the reaction temperature, 150 ml of water was added to decompose excess acetic anhydride. Then, extract with cyclohexane at 50° C. in portions, 300 ml each time, and extract 5 times in total. The extracts were combined, cyclohexane was recovered under reduced pressure, and 40 g of the product was obtained by recrystallization from ethanol, with a content of 97.1% and a yield of 78%.
Embodiment 2
[0021] Synthesis and purification of embodiment 2 compound 2a
[0022]
[0023] Put 50 g of the crude product of pregna-16-en-20-one-3-ol (content 85%) in a dry 1 liter three-neck reaction flask, add 100 ml of acetic anhydride, and heat to dissolve. After 45 min at reflux temperature, the acetylation reaction was complete. After cooling the reaction temperature, 100 ml of water was added to decompose excess acetic anhydride. Then, extract with 60°C hot cyclohexane in batches, 250ml each time, and extract 6 times in total. The extracts were combined, cyclohexane was recovered under reduced pressure, and then recrystallized with ethanol to obtain 37.4 g of the product with a content of 96.5% and a yield of 75%.
Embodiment 3
[0024] Synthesis and purification of embodiment 3 compound 2a
[0025]
[0026] Put 50 g of the crude product of pregna-16-en-20-one-3-ol (content 82%) in a dry 1 liter three-neck reaction flask, add 80 ml of acetic anhydride, and heat to dissolve. After reacting at 120°C for 1.5h, the acetylation reaction was complete. After cooling the reaction temperature, 100 ml of water was added to decompose excess acetic anhydride. Then, use 60°C hot petroleum ether to extract in portions, 260ml each time, and extract 7 times in total. The extracts were combined, petroleum ether was recovered under reduced pressure, and 35.7 g of the product was obtained by recrystallization with methanol, with a content of 96.2% and a weight yield of 74%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com