Preparation method of tannic acid curing chitosan microsphere heavy metal ion adsorbent
A technology of chitosan microspheres and heavy metal ions, applied in chemical instruments and methods, other chemical processes, etc., can solve the problems of less repeated use of adsorbents and unsatisfactory recovery of adsorbent performance, and achieve desorption Easy, excellent performance, large adsorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
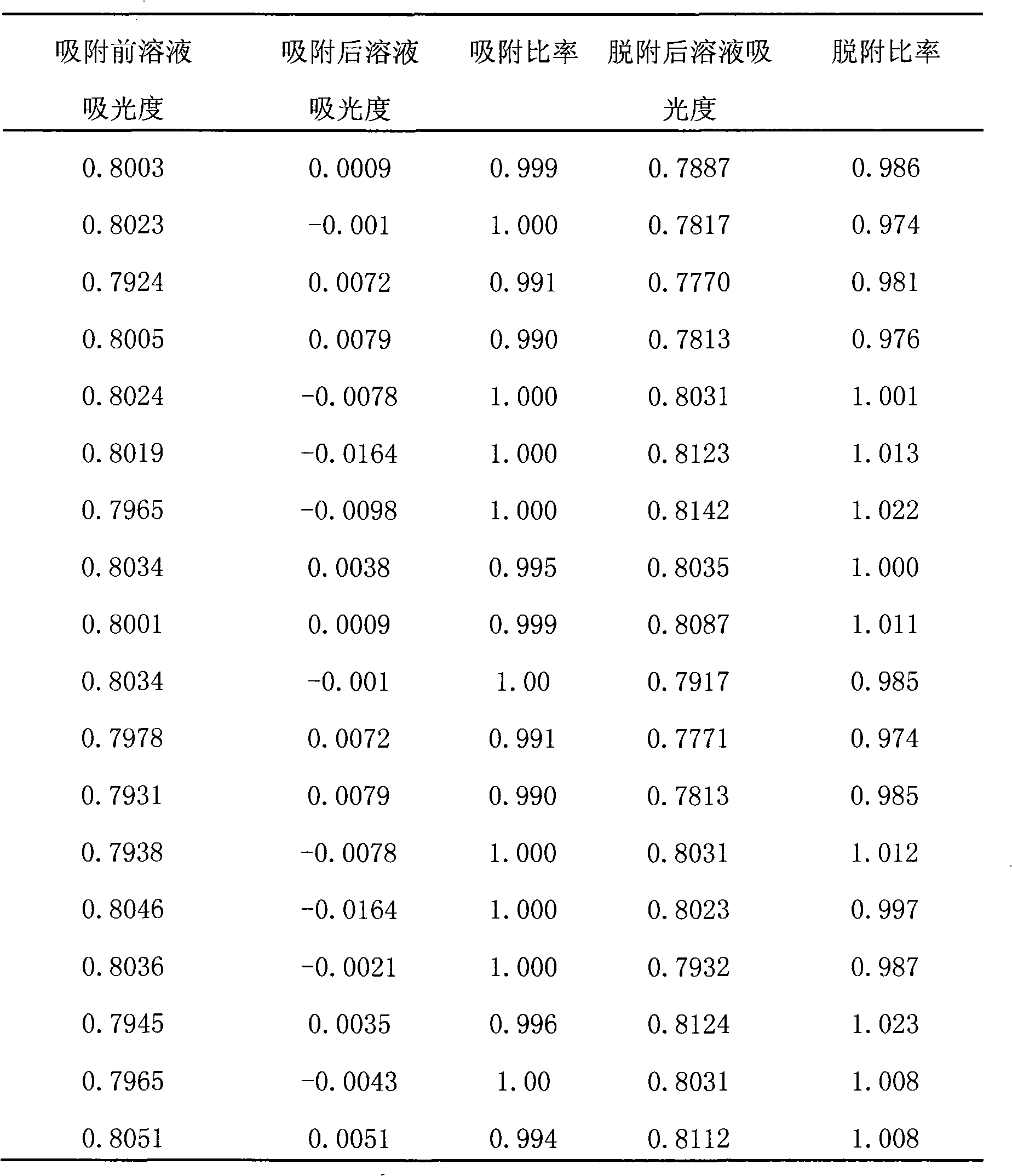
[0017] Weigh 5g of chitosan and dissolve it in 120mL of 3% acetic acid aqueous solution by mass fraction, add 0.7g of PEG and stir to make it fully dissolved, then add 150mL of liquid paraffin and 2mL of emulsifier and continue to stir to make the system uniform. Add 25mL formaldehyde and react for 3h. The mixture was poured into a large amount of ethanol and NaOH (10%wt%) mixed solution with a volume ratio of 1:1. Suction filtration after stirring, repeated washing until neutral, to obtain wet state chitosan microspheres. Weigh 7 g of tannic acid in 100 mL of distilled water, adjust the pH to 7 to make it a clear solution, add 10 g of wet chitosan microspheres that have not been cross-linked by epichlorohydrin, and react in a water bath at 50 ° C for 6 h. Filter and rinse with distilled water. Put the product into 300 mL of epichlorohydrin with a concentration of 0.06 mol / L, adjust the pH to 10, react at room temperature for 1 h, and react in a water bath at 50° C. for 4 h....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com