Process for producing cyclohexylbenzene
A cyclohexylbenzene and process technology, which is applied in the field of preparation of cyclohexylbenzene, can solve the problem of expensive recovery of feed materials, and achieve the effect of high selectivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1: catalyst preparation
[0074] The catalyst was prepared by co-pelleting the Pd catalyst with the MCM-49 catalyst. The Pd catalyst was prepared by impregnating 5 grams of gamma alumina with palladium nitrate solution and then calcining the Pd impregnated alumina at 350°C in air for 3 hours. The Pd loading on alumina was 0.3 wt%. The MCM-49 catalyst was prepared by crushing an extrudate having 80 wt% MCM-49 and 20 wt% alumina to particles 0.042 cm (1 / 60") or finer. The Pd / Al 2 o 3 Catalyst with crushed MCM-49 / Al 2 o 3 Mixed in a 1 :3 weight ratio and then pelletized using a hand press at 138 MPag (20,000 psig) pressure to form co-pelletized catalyst. The catalyst was then sized for testing based on a mesh opening of 0.250mm-0.149mm (60-100 mesh).
Embodiment 2
[0075] Example 2: Hydroalkylation of Benzene
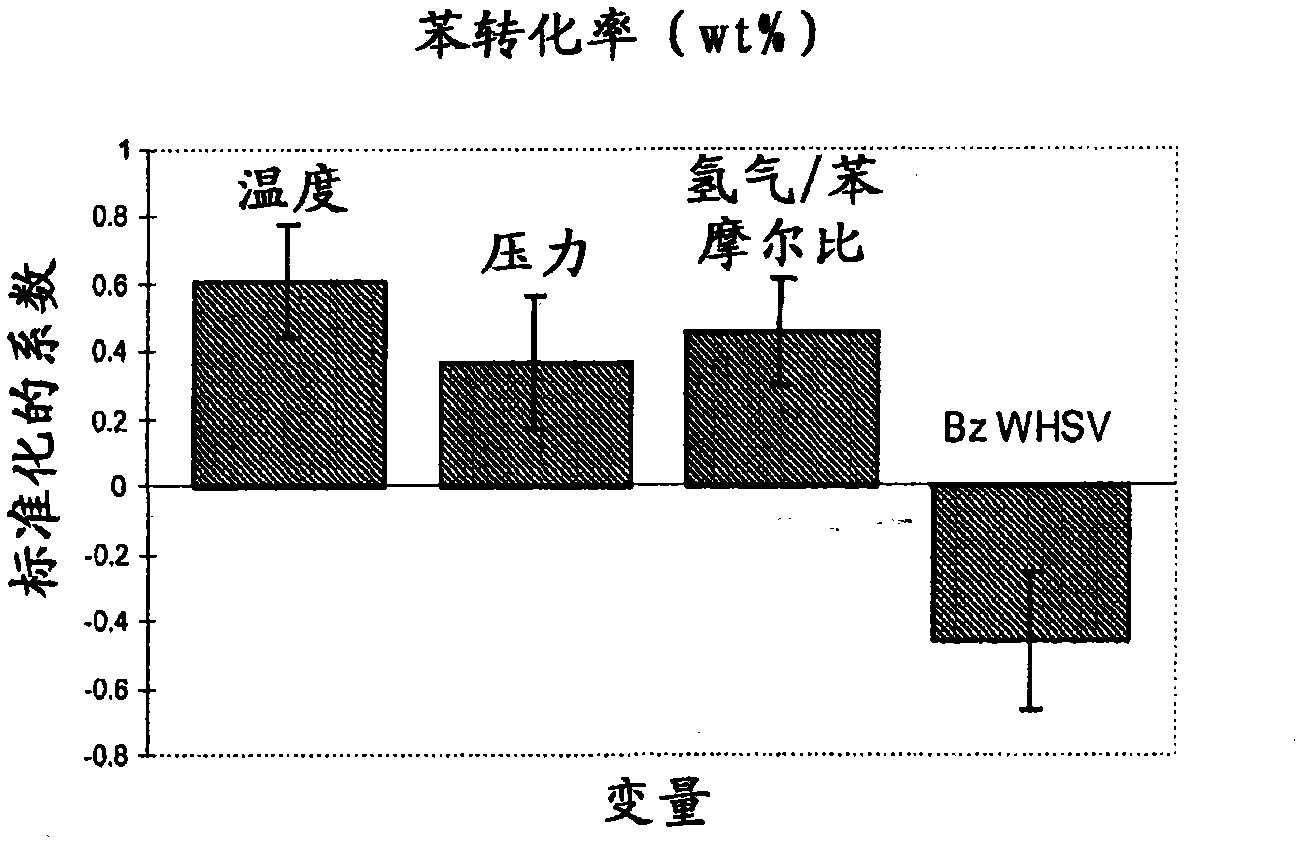
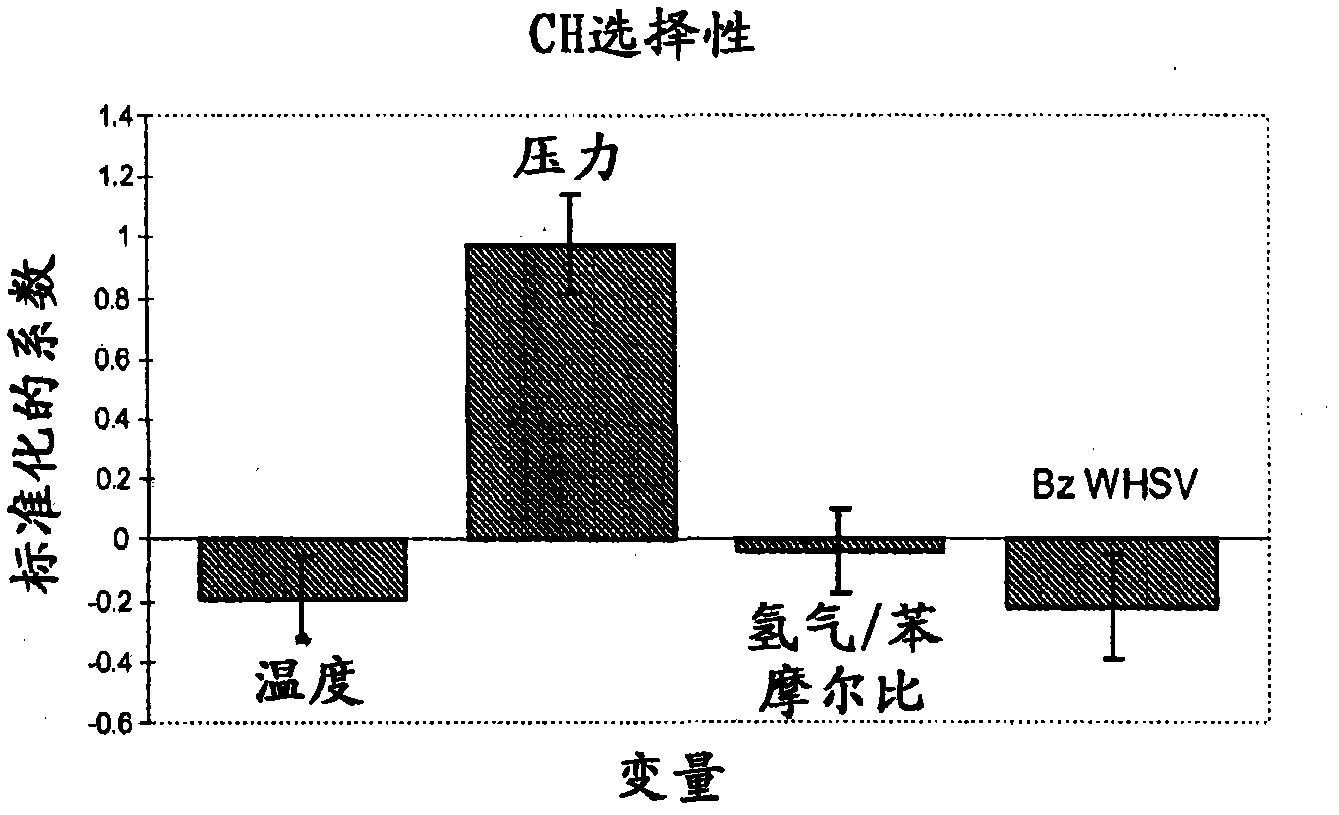
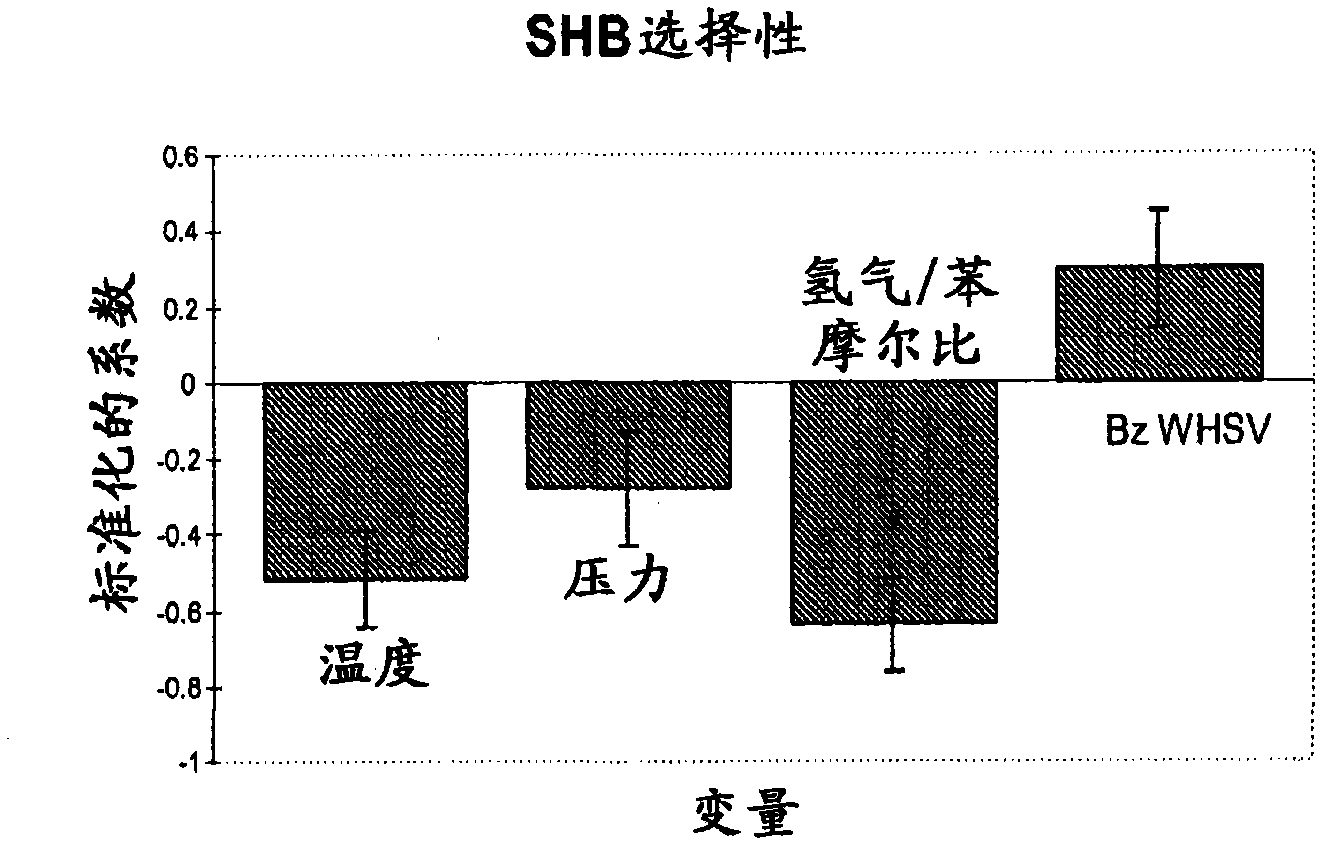
[0076] 8 grams of the catalyst prepared in Example 1 were loaded into a stainless steel fixed-bed microreactor. The reactor had a 1.27 cm (1 / 2 inch) internal diameter with a 0.32 cm (1 / 8 inch) thermowell in the center through the catalyst bed. The catalyst was pretreated with 100 cc / min hydrogen at 300 °C and 1 atm for 2 h. After cooling to 155°C in hydrogen, benzene was fed into the reactor via a syringe pump at 60 cc / hour for 1 hour while increasing the reactor pressure to 1034 kPag (150 psig). The benzene feed rate was then reduced to 0.52 WHSV and the hydrogen / benzene molar ratio was adjusted to 1.28. Liquid product was collected in a cold product trap and analyzed off-line. Catalyst performance was evaluated using different experimental conditions by varying four process variables. Table 2 shows these process variables and their ranges. CHB production was investigated using a total of 36 experimental conditions.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com