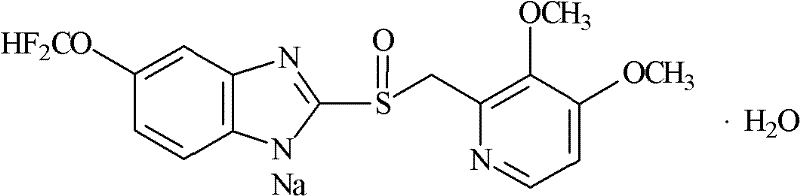
S-pantoprazole sodium composite for injection and preparation method thereof
A technology of pantoprazole sodium and its composition, which is applied in the field of pharmaceutical preparation, can solve problems such as unresolved technical defects, and achieve the effects of avoiding hidden dangers, avoiding scrapping, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
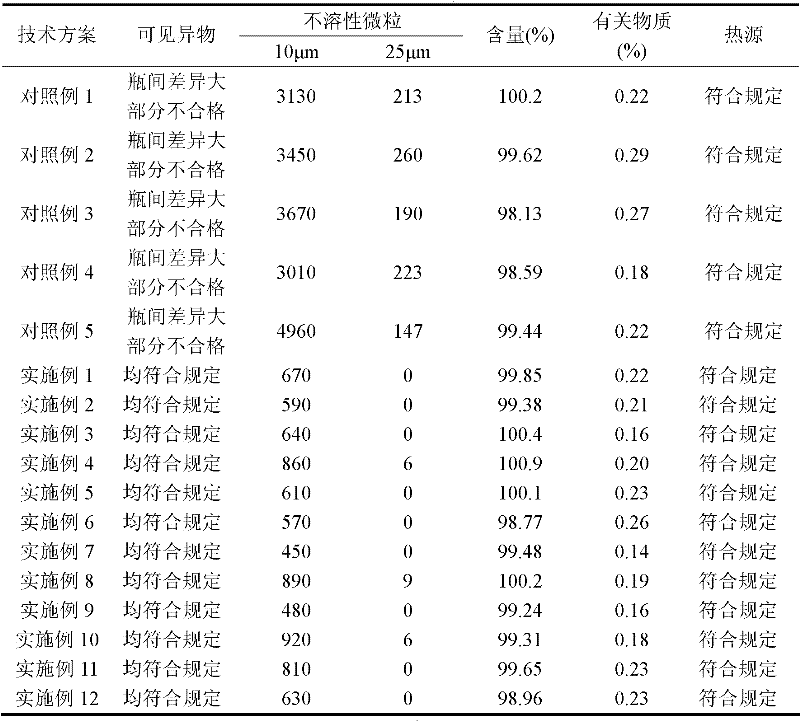
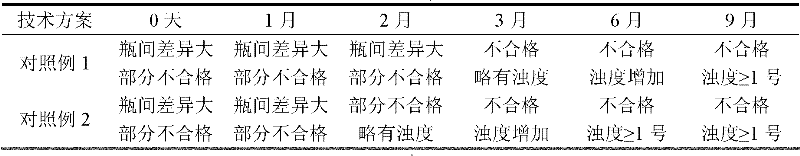
Image
Examples
Embodiment 1
[0083] Embodiment 1 prepares the S-pantoprazole sodium composition for injection
[0084] prescription:
[0085] S-pantoprazole sodium 846g
[0086] EDTA 8.46g
[0087] Mannitol 1Kg
[0088] Add water for injection to 20Kg
[0089] 1) Preparation of medicinal solution: Weigh S-pantoprazole sodium, mannitol and ethylenediaminetetraacetic acid according to the prescription and put them in the preparation tank, add water for injection to 20Kg, stir to dissolve and mix evenly, and adjust the pH value of the solution to 12.0 ;
[0090] 2) Activated carbon finishing:
[0091] a. Weigh 10g of activated carbon, add 500g of water for injection, stir to disperse evenly, adjust the pH value of the solution to 12.0, heat to 65°C and keep it warm for 30 minutes, and transfer the activated carbon suspension to a titanium rod filter for decarbonization while it is hot;
[0092] B, then rinse the gac collected by the titanium rod filter with water for injection until the pH value is les...
Embodiment 2
[0098] Embodiment 2 prepares the S-pantoprazole sodium composition for injection
[0099] prescription:
[0100] S-pantoprazole sodium 846g
[0101] Calcium Disodium EDTA 42.3g
[0102] Add water for injection to 60Kg
[0103] 1) Preparation of medicinal solution: Weigh S-pantoprazole sodium and calcium disodium edetate according to the prescription and put them in the preparation tank, add water for injection to 60Kg, stir to dissolve and mix evenly, and adjust the pH value of the solution to 11.0 ;
[0104] 2) Activated carbon finishing:
[0105] a. Weigh 300g of activated carbon, add 15Kg of water for injection, stir to disperse evenly, adjust the pH value of the solution to 11.0, heat to 66°C and keep it warm for 30 minutes, and transfer the activated carbon suspension to a titanium rod filter for decarbonization while it is hot;
[0106] B, then rinse the gac collected by the titanium rod filter with water for injection until the pH value is less than 8;
[0107] c....
Embodiment 3
[0112] Embodiment 3 prepares the S-pantoprazole sodium composition for injection
[0113] prescription:
[0114] S-pantoprazole sodium 1.268Kg
[0115] Calcium Disodium EDTA 25.36g
[0116] Add water for injection to 100Kg
[0117] 1) Preparation of medicinal solution: Weigh S-pantoprazole sodium and edetate calcium disodium according to the prescription and put them in the preparation tank, add water for injection to 100Kg, stir to dissolve and mix evenly, and adjust the pH value of the solution to 10.5 ;
[0118] 2) Activated carbon finishing:
[0119] a. Weigh 300g of activated carbon, add 15Kg of water for injection, stir to disperse evenly, adjust the pH value of the solution to 10.5, heat to 63°C and keep it warm for 30 minutes, and transfer the activated carbon suspension to a titanium rod filter for decarbonization while it is hot;
[0120] B, then rinse the gac collected by the titanium rod filter with water for injection until the pH value is less than 8;
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com