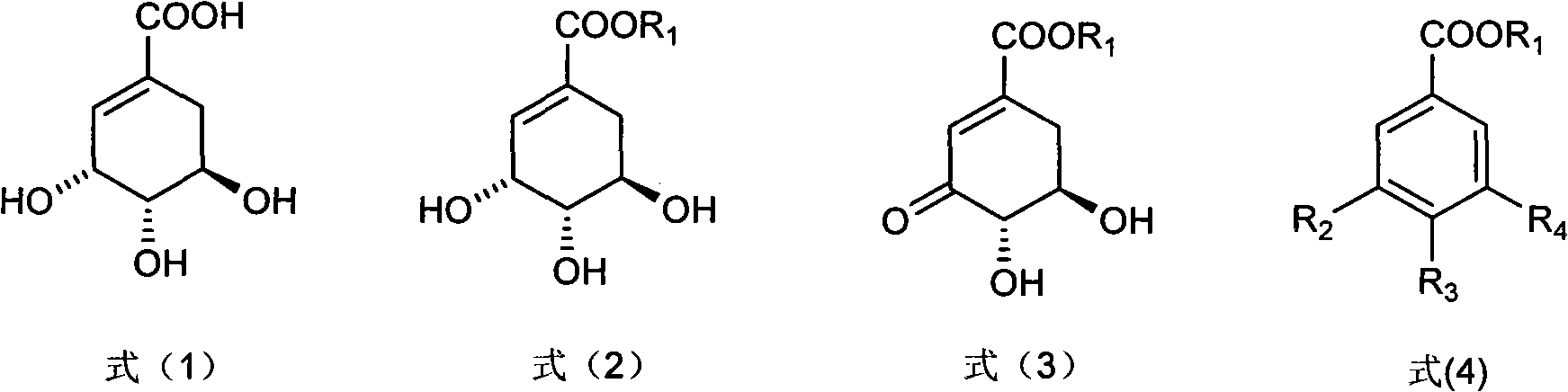
Method for preparing epicatechol gallate and protocatechuic acid ester compounds from natural shikimic acid
A technology of gallate and catechin, which is applied in the preparation of organic compounds, preparation of carboxylic acid esters, chemical instruments and methods, etc., to achieve the effect of low equipment requirements, mild reaction conditions, and increase farmers' income
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
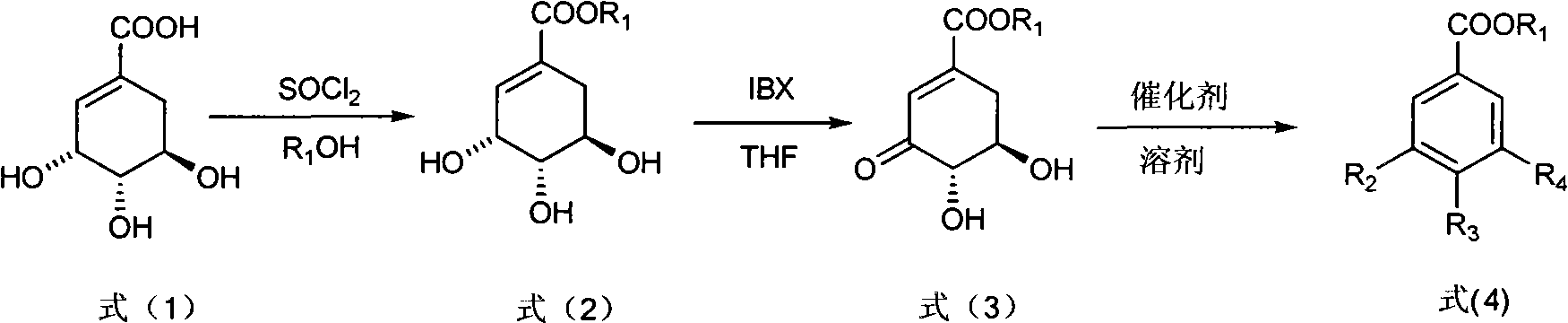
[0026] Step 1: Add shikimic acid (17.4g, 0.1mol) and anhydrous methanol (150mL, 3.7mol) in a 250mL three-necked round-bottomed flask, and gradually add chlorinated oxychloride dropwise under sufficient stirring to control the internal temperature of the reaction solution at 10°C Sulfone (15 mL, 0.2 mol). After the dropwise addition was completed, the internal temperature was controlled at 40° C., and the reaction was carried out for 3 hours. Concentrate to obtain a viscous liquid, which is recrystallized from ethyl acetate to obtain 14.76 g of white powdery solid methyl shikimate, yield: 78.5%. m.p.112~113℃. (c=0.2, MeOH); 1 H NMR (CD 3 COCD 3 , 400MHz) δ6.73(m, 1H, 2-H), 4.38(m, 1H, 3-H), 4.02(s, 1H, 4-OH D 2 O exchangeable), 4.00 (brs, 2H, 3, 5-OH D 2 O exchangeable), 3.69 (s, 3H, OCH 3 ), 3.85(m, 1H, 5-H), 3.68(m, 1H, 4-H), 2.64(dd, J 1 =17.6Hz,J 2 = 4.4Hz, 1H, 6α-H), 2.18(dd, J 1 =17.6Hz,J 2 =6.8Hz, 1H, 6β-H).MS(EI), m / z(%): 188(M + ), 170(M + -H 2 O), 157 (...
Embodiment 2
[0030] Step 1: Same as Example 1.
[0031] Step 2: Put methyl shikimate (7.52g, 40mmol), IBX (13.44g, 48mmol) and 160mL tetrahydrofuran into a reaction flask, heat to 67°C, and stir for 1h. Filtration and concentration gave a light yellow solid. Recrystallized from ethyl acetate-petroleum ether to obtain 3.84 g of white fine needle crystals of methyl 3-dehydroshikimate, yield: 51.6%.
[0032] Step 3: Methyl 3-dehydroshikimate (0.744 g, 4 mmol), Cu(OAc) 2 ·H 2 O (1.60g, 8mmol) and AcOH / H 2 O (2:1, V / V) 50mL was put into the reaction flask, heated to 50°C, and stirred for 26h. Filtration, concentration, extraction with ethyl acetate, combined organic layers were dried over anhydrous magnesium sulfate, concentrated, and then recrystallized with water to obtain 0.58 g of white solid methyl gallate, yield: 78%.
Embodiment 3
[0034] Step 1: Same as Example 1.
[0035] Step 2: Put methyl shikimate (7.52g, 40mmol), IBX (13.44g, 48mmol) and 160mL tetrahydrofuran into a reaction flask, heat to 50°C, and stir for 6h. Filtration and concentration gave a light yellow solid. Recrystallized from ethyl acetate-petroleum ether to obtain 4.17 g of white fine needle crystals of methyl 3-dehydroshikimate, yield: 56.0%.
[0036] Step 3: Methyl 3-dehydroshikimate (0.744g, 4mmol), ZnO (0.163g, 0.8mmol) and AcOH / H 2 50 mL of O (6:1, V / V) was put into the reaction flask, and heated to 50° C. for 16 h. Concentrate, extract with ethyl acetate, combine the organic layers, dry over anhydrous magnesium sulfate, concentrate, and recrystallize with water to obtain 0.61 g of methyl protocatechuate as a white solid, yield: 90.5%. m.p.134~135℃. 1 HNMR (CD 3 COCD 3 , 400MHz) δ8.50 (brs, 2H, 3, 4-OH), 7.48 (d, J=2.0, 1H, 2-H), 7.43 (dd, J 1 =8.0Hz,J 2 =2.0Hz, 1H, 6-ArH), 6.89(d, J 1 =8.4Hz, 1H, 5-ArH).MS(EI), m / z(%): 16...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com