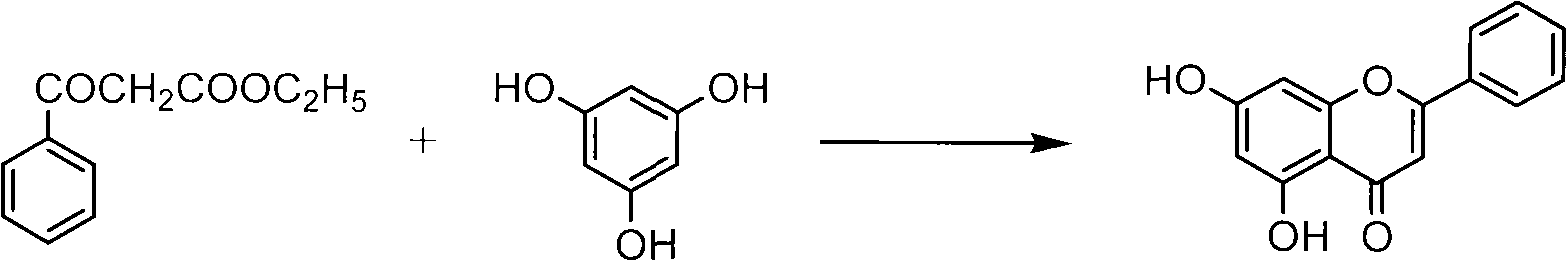
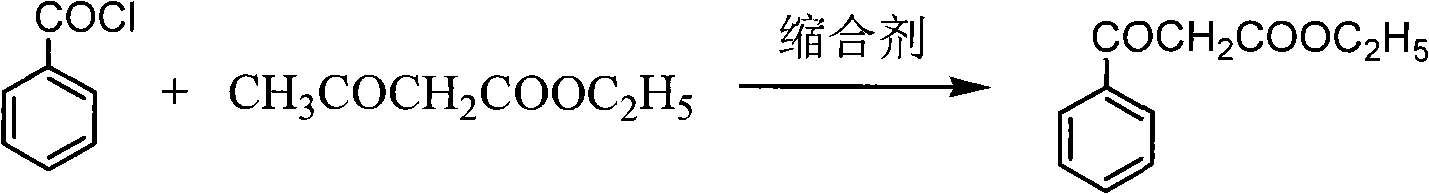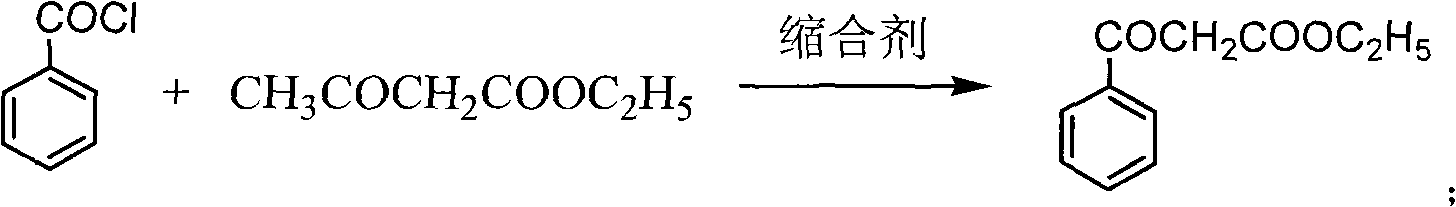Method for synthesizing 5,7-dihydroxyflavone
A technique for the synthesis of dihydroxyflavones, which is applied in the field of synthesis of flavonoids, can solve problems such as large environmental pollution, and achieve the effects of less environmental pollution, rich sources of raw materials, and short reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of ethyl benzoylacetate
[0020] Put drinking water into the reaction kettle, pump in 100kg of ethyl acetoacetate, cool down to 5±5°C, slowly add sodium hydroxide solution dropwise, and keep warm. At the same time, start to add benzoyl chloride dropwise, keep the temperature in the kettle not exceeding 10°C, and keep the pH>11. After the dropwise addition, keep warm for 5 hours. Ammonium chloride was added, and the reaction was maintained at 25±5°C for 8 hours. After completion of the reaction, after-treatment, about 150kg of ethyl benzoyl acetate crude distillate was obtained. Distill once with Roots pump high vacuum, obtain about 133kg light yellow oily liquid. HPLC >97%, yield 90%.
Embodiment 2
[0022] Preparation of ethyl benzoylacetate
[0023] Put drinking water into the reaction kettle, pump in 100kg of ethyl acetoacetate, cool down to 5±5°C, slowly add potassium hydroxide solution dropwise, and keep warm. At the same time, start to add benzoyl chloride dropwise, keep the temperature in the kettle not exceeding 10°C, and keep the pH>11. After the dropwise addition, keep warm for 5 hours. Ammonium chloride was added, and the reaction was maintained at 25±5°C for 8 hours. After completion of the reaction, after-treatment, about 145kg of ethyl benzoyl acetate crude distillate was obtained. Distill once with Roots pump high vacuum, obtain about 125kg light yellow oily liquid. HPLC>97%, yield 84.5%.
Embodiment 3
[0025] Preparation of ethyl benzoylacetate
[0026] Put an appropriate amount of methanol into the reaction kettle, pump in 100kg of ethyl acetoacetate, cool down to 5±5°C, slowly add sodium hydroxide solution dropwise, and keep warm. At the same time, start to add benzoyl chloride dropwise, keep the temperature in the kettle not exceeding 10°C, and keep the pH>11. After the dropwise addition, keep warm for 5 hours. Add ammonium chloride aqueous solution, and keep the reaction at 25±5°C for 8 hours. After completion of the reaction, after-treatment, about 120kg of ethyl benzoyl acetate crude distillate was obtained. Distill once with Roots pump high vacuum, obtain about 108kg light yellow oily liquid. HPLC >97%, yield 73%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com