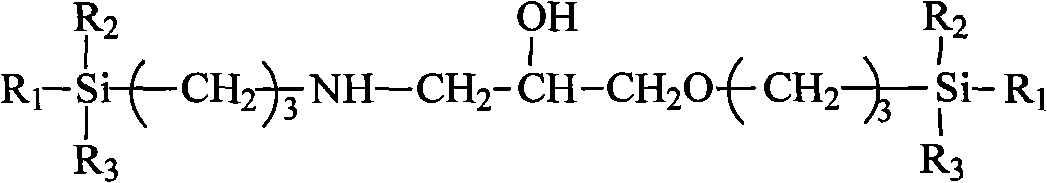Light-cured resin with low water absorption and preparation method thereof
A light-curing resin, low water absorption technology, applied in the field of light-curing materials, can solve the problems of poor product compatibility, poor control of the reaction, small molecular weight of the product, etc., and achieves a structure with regular structure, easy control and narrow molecular weight distribution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Weigh 10g of γ-aminopropyltriethoxysilane (KH-550) and add it to 11g of γ-(2,3-epoxypropoxy)propyltrimethoxysilane (KH-560) in a three-necked flask, mix well Then heat up to 70°C and react for 2 hours to obtain a modified silane chain extender containing secondary amino and hydroxyl functional groups, whose structural formula is:
[0031]
[0032] Among them, R 1 , R 2 , R 3 stands for CH 3 -, C 2 h 5 O- or CH 3 O-;
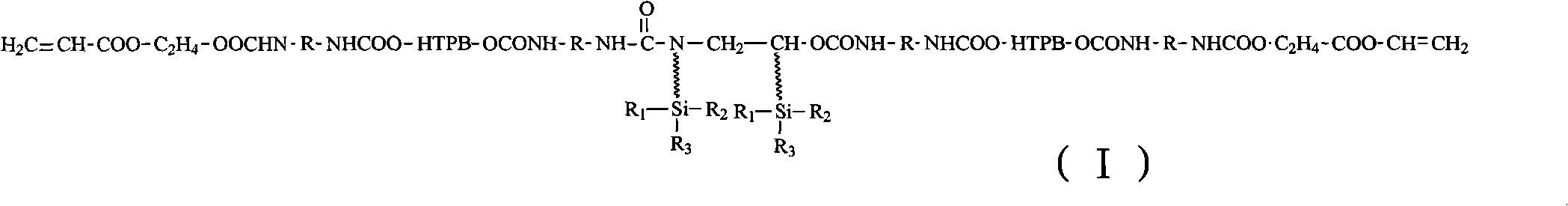
[0033] Vacuumize 100g of HTPB at 110°C (vacuum degree ≤ -0.08MPa) for 2 hours to remove the doped water; then cool down to 50°C, add 28.5g of TDI, and heat up to 85°C for 2 hours after the temperature is constant , and stop heating until the NCO content reaches the design value, that is, a polyurethane prepolymer with NCO groups at both ends is obtained, and its structural formula is: ,
[0034] Wherein, R represents toluene diisocyanate;
[0035] After mixing 100g of polyurethane prepolymer 1 and 5.6g of modified silane chain extender, react...
Embodiment 2
[0041]Weigh 10g of γ-aminopropyltriethoxysilane (KH-550) and add it to 11g of γ-(2,3-epoxypropoxy)propylmethyldimethoxysilane (WD-61) in a three-necked flask , after mixing evenly, heat up to 50°C and react for 3 hours to obtain a modified silane chain extender containing secondary amino and hydroxyl functional groups, which is the same as in Example 1;
[0042] Vacuumize 100g of HTPB at 100°C (vacuum degree ≤ -0.08MPa) for 3 hours to remove the doped water; then cool down to 60°C, add 24g of IPDI, and heat up to 80°C for 3 hours after the temperature is constant. Stop heating until the NCO content reaches the design value, and obtain polyurethane prepolymer 1 with NCO groups at both ends. Its structural formula is the same as that of Example 1, and R represents isophorone diisocyanate.
[0043] Mix 100g of polyurethane prepolymer 1 and 4.6g of modified silane chain extender, and react at 70°C for 3 hours to obtain polyurethane prepolymer 2 with NCO groups at both ends. Its st...
Embodiment 3
[0046] Weigh 10g of γ-aminopropylmethyldiethoxysilane (WD-57) and add it to 10g of γ-(2,3-epoxypropoxy)propyltrimethoxysilane (KH-560) in a three-necked flask, After mixing evenly, heat up to 90°C and react for 1 hour to obtain a modified silane chain extender containing secondary amino and hydroxyl functional groups, which is the same as in Example 1;
[0047] Vacuumize 100g of HTPB at 115°C (vacuum degree ≤ -0.08MPa) for 1.5 hours to remove the doped water; then cool down to 40°C, add 31g of MDI, and heat up to 90°C for 1 hour after the temperature is constant. Stop heating until the NCO content reaches the design value, and obtain polyurethane prepolymer 1 with NCO groups at both ends. Its structural formula is the same as that of Example 1, and R represents diphenylmethane diisocyanate.
[0048] After mixing 100g of polyurethane prepolymer 1 and 4.9g of modified silane chain extender, react at 90°C for 1 hour to obtain polyurethane prepolymer 2 with NCO groups at both ends...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com