Method for preparing molybdenum pentachloride
A technology of molybdenum pentachloride and carbon tetrachloride, applied in the direction of molybdenum halide, etc., can solve the problems of high toxicity and strong corrosion of chlorine gas, difficulty in ensuring safety, high energy consumption in production, etc., and achieve single product phase structure and high production efficiency. The effect of low cost and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
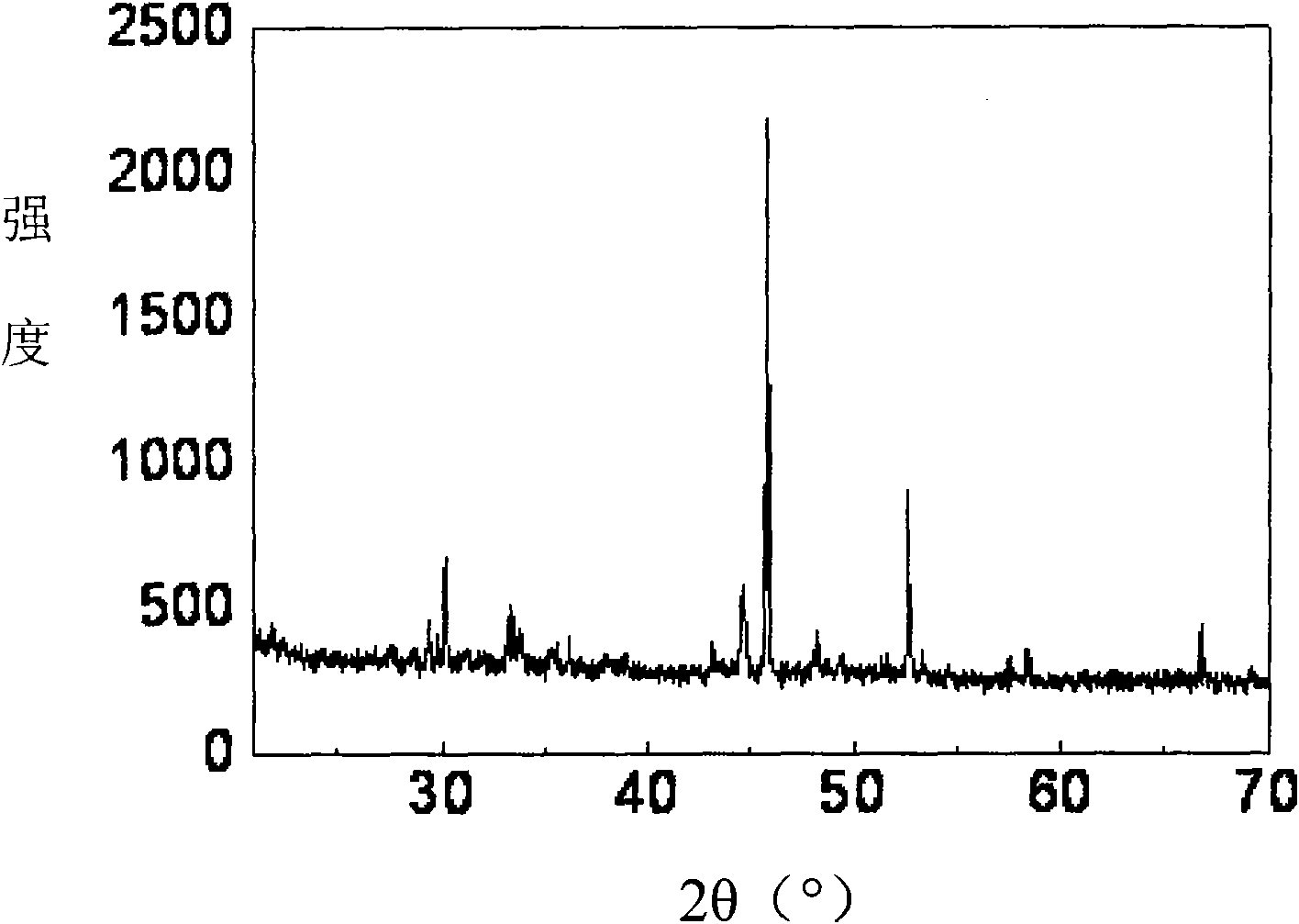
[0016] 10 g of molybdenum trioxide and 37.5 ml of dehydrated carbon tetrachloride (mass ratio 1:6) were put into a round-bottomed stainless steel reaction bottle for mixing, and the reaction bottle was degassed and then the valve was closed for sealing. Then submerge the entire reaction bottle in an oil bath and heat it to 150° C., keep it for 12 hours, and take it out after cooling. At this time, the reaction solution changed from colorless to brown-red, and black needle-like crystals were precipitated. Open reaction bottle valve and emit reaction gas, reaction solution is poured in evaporator, remove carbon tetrachloride by evaporation and promptly obtain black crystal, the phase structure analysis that carries out through X-ray diffraction confirms that this black crystal is molybdenum pentachloride (see attached figure 1 ). The product weighed 8.2 g, and the yield was 43%.
Embodiment 2
[0018] 10 g of molybdenum trioxide and 30 ml of dehydrated carbon tetrachloride (mass ratio 1:5) were put into a round-bottomed stainless steel reaction bottle for mixing, and the reaction bottle was degassed and then the valve was closed for sealing. Then all the reaction bottle was submerged in an oil bath and heated to 240° C., kept for 1 hour, and then taken out after cooling. At this time, the reaction solution changed from colorless to brown-red, and black needle-like crystals were precipitated. Open the valve of the reaction bottle to release the reaction gas, pour the reaction solution into the evaporator, remove carbon tetrachloride by evaporation to obtain 11.8 g of molybdenum pentachloride black crystals, and the product yield is 62%. Phase structure analysis result is the same as embodiment 1.
Embodiment 3
[0020] 10 g of molybdenum trioxide and 25 ml of dehydrated carbon tetrachloride (mass ratio 1:4) were put into a round-bottomed stainless steel reaction bottle for mixing, and the reaction bottle was degassed and then the valve was closed for sealing. Then put the whole reaction bottle into an oil bath and heat it to 180°C, keep it for 8 hours, take it out after cooling. At this time, the reaction solution changed from colorless to brown-red, and black needle-like crystals were precipitated. Open the valve of the reaction bottle to release the reaction gas, pour the reaction solution into the evaporator, and remove carbon tetrachloride by evaporation to obtain 14.8 g of molybdenum pentachloride black crystals, with a product yield of 78%. Phase structure analysis result is the same as embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com