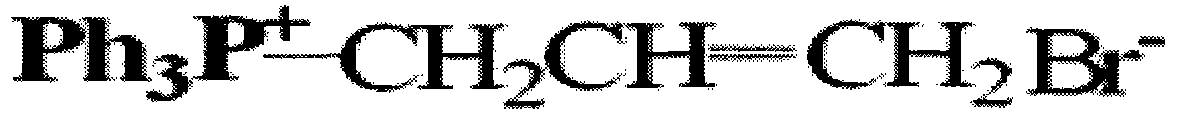Quaternary phosphonium salt as well as preparation method and application thereof
A quaternary phosphonium salt and stirring reaction technology, applied in the field of antibacterial agents, can solve the problems of complex synthetic organic antibacterial agent process, difficult preparation, high cost, and achieve the effects of less steps, wide application range and short use time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) This example uses halogenated olefin and trialkylphosphine in a molar ratio of 1.13:1.0. Weigh 8g of triphenylphosphine, dissolve it in 28ml of N,N-dimethylformamide, transfer the mixture into the dropping funnel; then measure 3ml of allyl bromide, dissolve it in 35ml of N,N- Dimethylformamide, and then transfer it to a three-necked flask; drop triphenylphosphine solution into the three-necked flask at a rate of drop / 2s, stir and react at 65°C for 10 hours to obtain a crude quaternary phosphonium salt ;
[0031] (2) Cool the crude quaternary phosphonium salt to 20° C., filter, enrich it, rinse with ether several times, and dry to obtain 6.97 g of the product, with a yield of 60%.
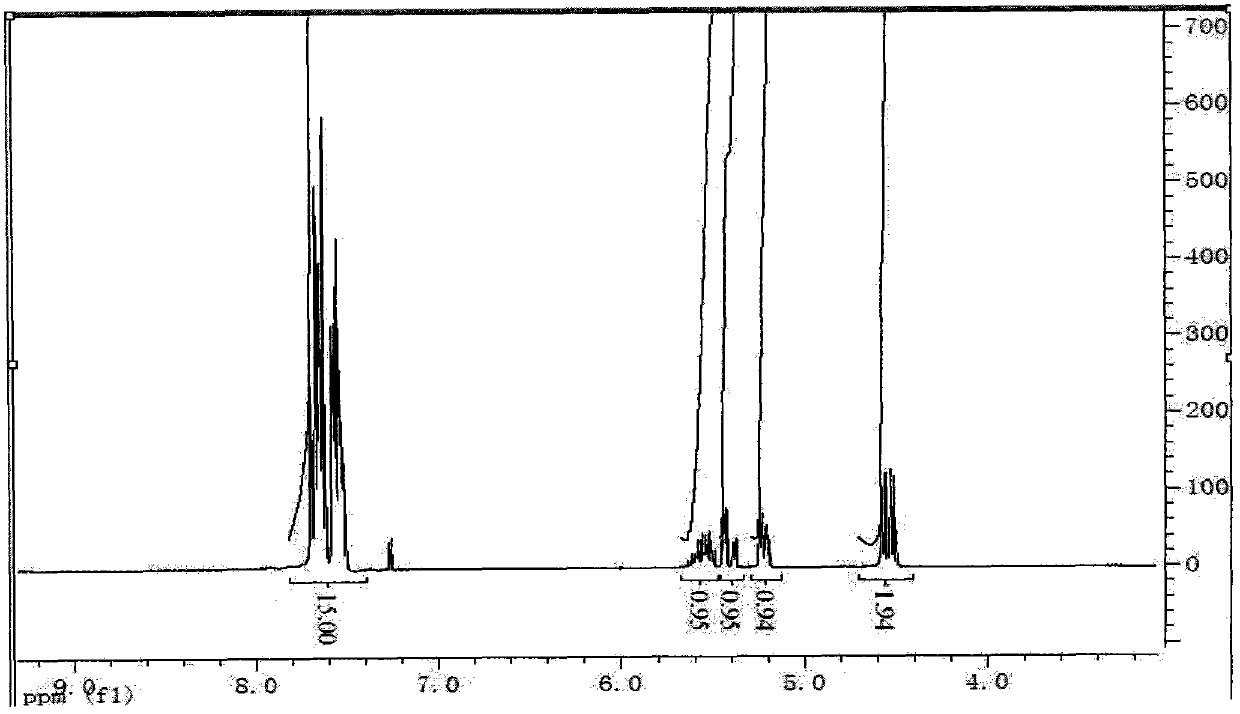
[0032] (3) Determine the structure of the product by infrared spectroscopy and proton nuclear magnetic resonance spectroscopy:
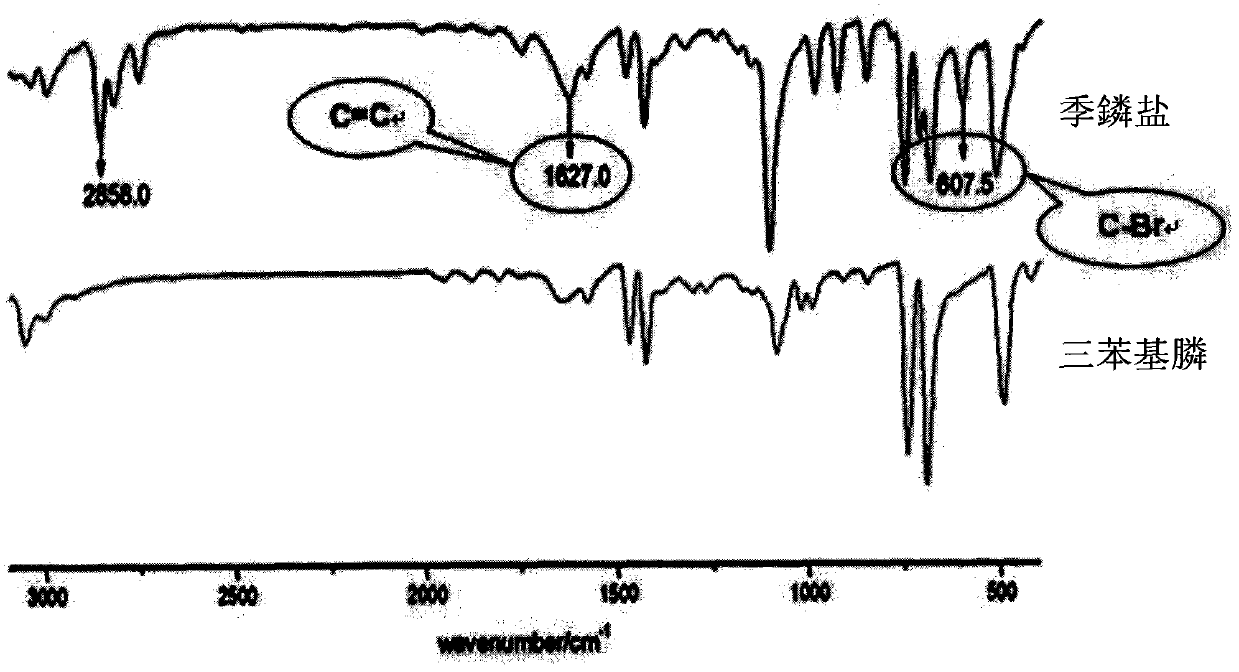
[0033] ① Determination of the structure of quaternary phosphonium salts by infrared spectroscopy
[0034] Depend on figure 1 The contrast infrared spectrum ...
Embodiment 2
[0038] (1) This example uses halogenated olefin and trialkylphosphine in a molar ratio of 1.5:1.0. Weigh 3.51g of tributylphosphine and dissolve in 12ml of tetrahydrofuran, and dissolve 1.7ml of methallyl chloride in 16ml of tetrahydrofuran, stir and react at 55°C for 12h to obtain the crude quaternary phosphonium salt;
[0039] (2) After the crude quaternary phosphonium salt product is cooled to 25° C., it is filtered, enriched, washed with petroleum ether several times, and dried to obtain the product.
[0040] (3) The infrared spectrum and proton nuclear magnetic resonance spectrum of the product are the same as in Example 1, illustrating that the structure of the product is as shown in formula I.
Embodiment 3
[0042] (1) This example uses a 1.13:1.0 haloalkene and trialkylphosphine. Weigh 8g of triphenylphosphine, dissolve it in 28ml of benzene, and transfer the mixture to the dropping funnel; then measure 3ml of 3,3-dimethylallyl bromide, dissolve it in 35ml of benzene, and transfer it to into the three-necked flask; drip the triphenylphosphine solution into the three-necked flask at the speed of drops / 2s, stir and react at 75°C for 7h, and obtain the crude quaternary phosphonium salt;
[0043] (2) Cool the crude quaternary phosphonium salt product to 30° C., filter, enrich it, rinse with ether several times, and dry to obtain the product.
[0044] (3) The infrared spectrum and proton nuclear magnetic resonance spectrum of the product are the same as in Example 1, illustrating that the structure of the product is as shown in formula I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com