Three-dimensional mesh nano porous palladium-ruthenium electrode material for fuel cell and preparation method thereof
A three-dimensional network, nanoporous technology, applied in the direction of battery electrodes, circuits, electrical components, etc., can solve the problems that limit the practical application of direct formic acid fuel cells, and achieve good catalytic activity, large specific surface area, and high electrochemical activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The titanium sheet (5 mm × 10 mm × 1.0 mm) was rinsed with water three times, then heated in 18% hydrochloric acid at 85 °C for 10 min to remove the oxide layer on the titanium sheet, and then ultrasonically cleaned for 10 min, the treated titanium Tablets were placed in a hydrothermal reaction kettle, and 10 mL, 5 mmol L -1 PdCl2, 0.05 mmol EDTA and 1 mL 10% HCHO were placed in an infrared drying oven at 180 °C for 10 h, and after cooling to room temperature, the sample was taken out and dried at 100 °C for 10 min to obtain a three-dimensional network nano-Pd electrode.
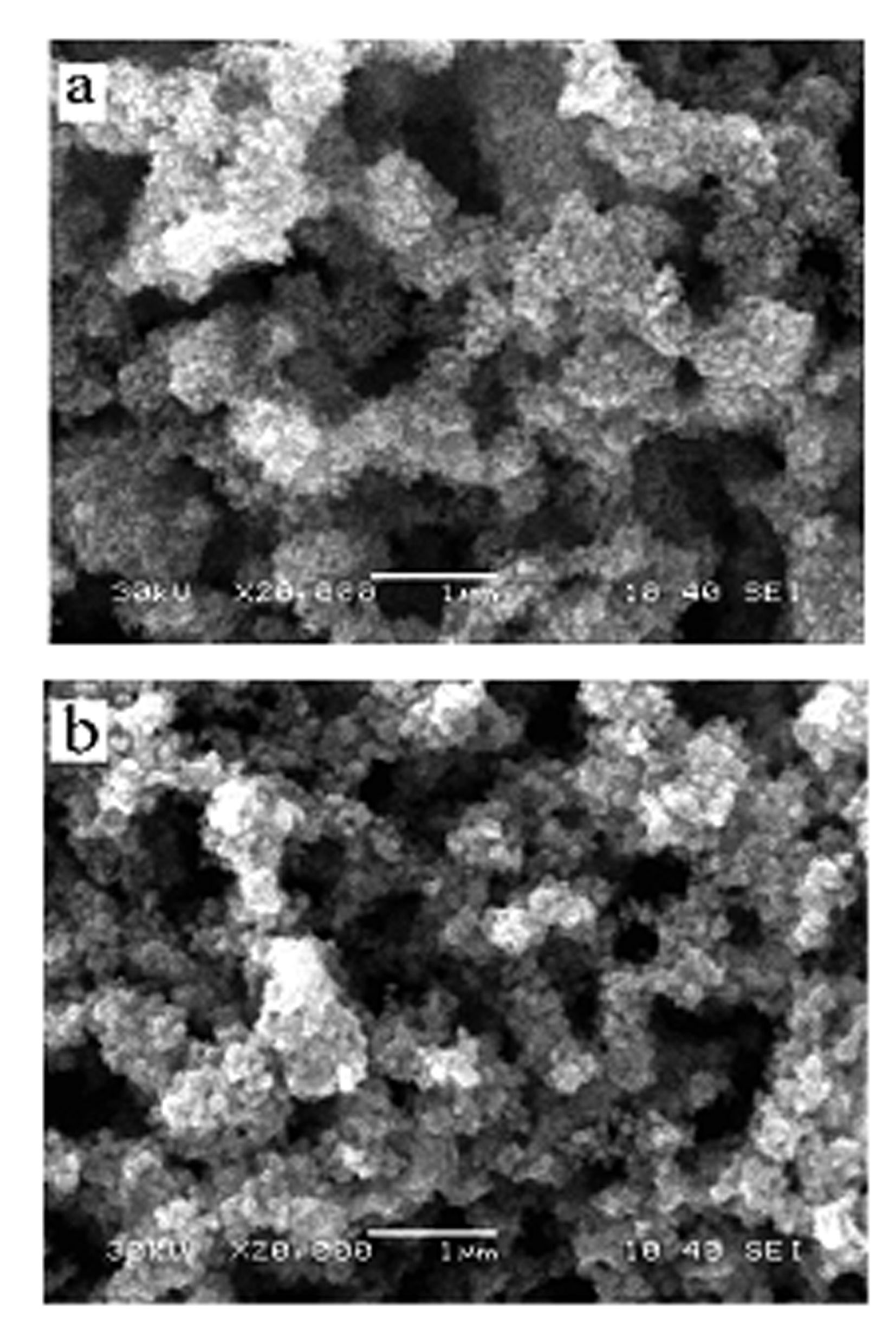
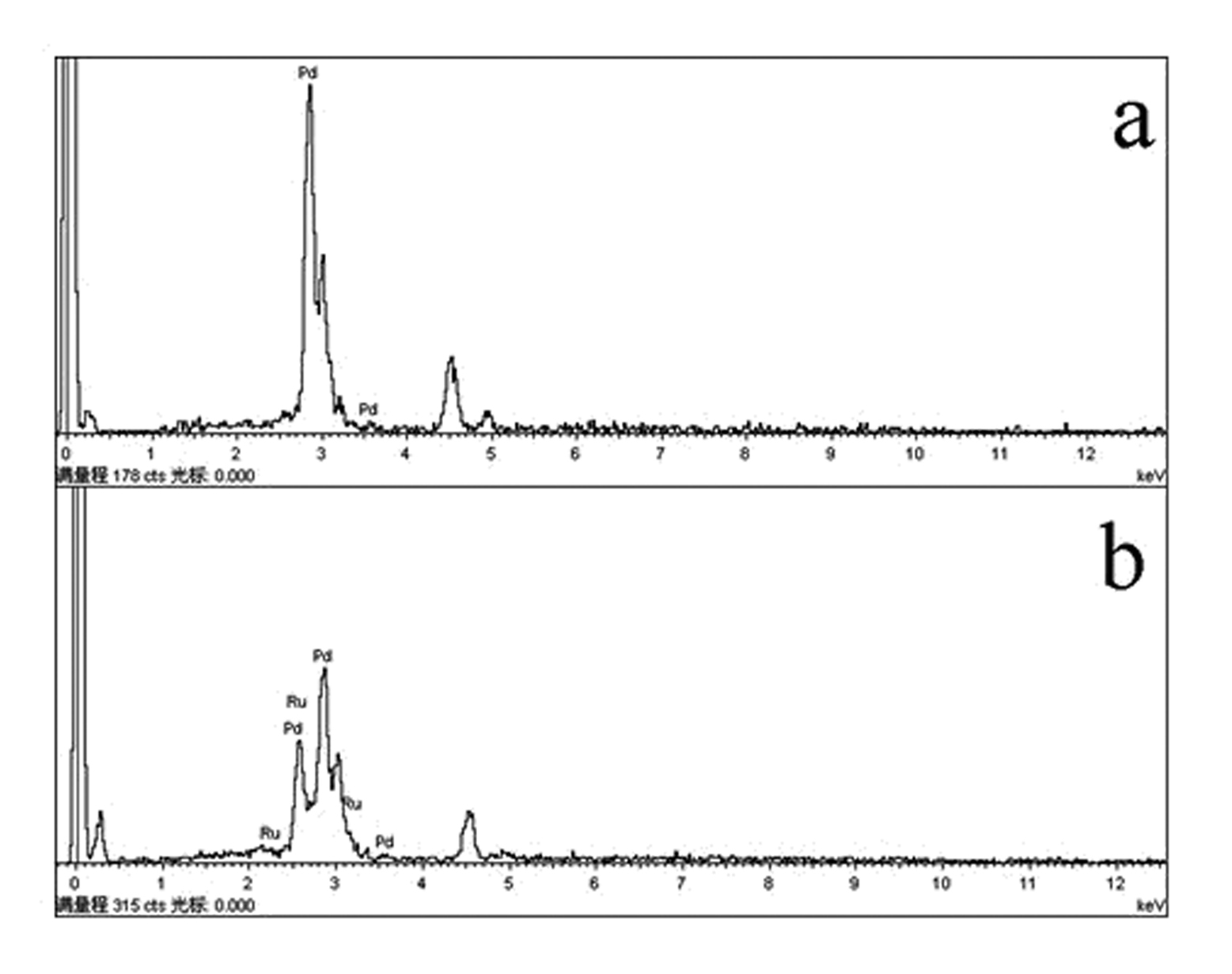
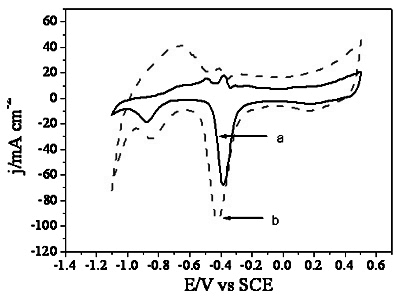
[0018] The surface morphology of the prepared electrode was measured by a JSM6380LV scanning electron microscope ( figure 1 a) and energy spectrum analysis ( figure 2 a). The electrochemical test was carried out on atoLABPGSTA30 / FRA, a three-chamber glass electrolytic cell, the working electrode was a three-dimensional network nano-Pd electrode, the counter electrode was a large-area Pt electro...
Embodiment 2
[0021] Rinse the titanium sheet (5 mm×10 mm×1.0 mm) with water three times, and then heat it in 18% hydrochloric acid at 85°C for 10 minutes to remove the oxide layer on the surface of the titanium sheet, and put the treated titanium sheet in the hydrothermal reaction In the kettle, add 10 mL, 5 mmol L -1 PdCl 2 , 0.05 mmol EDTA, RuCl 3 and 1 mL of 10% HCHO, and then placed in an infrared drying oven at 180 °C for 10 h. After cooling to room temperature, the sample was taken out and dried at 100 °C for 10 min to obtain a three-dimensional network nano-PdRu electrode.
[0022] The surface morphology of the prepared electrode was measured by a JSM6380LV scanning electron microscope ( figure 1 b) and energy spectrum analysis ( figure 2 b). The electrochemical test was carried out on atoLABPGSTA30 / FRA, a three-chamber glass electrolytic cell, the working electrode was a three-dimensional network nano-PdRu electrode, the counter electrode was a large-area Pt electrode, an...
Embodiment 3
[0025] Rinse the titanium sheet (5 mm×10 mm×1.0 mm) with water three times, then heat it in 18% hydrochloric acid at 80°C for 10 min to remove the oxide layer on the surface of the titanium sheet, and put the treated titanium sheet in the hydrothermal reaction In the kettle, add 12 mL, 5 mmol L -1 PdCl 2 , 0.06 mmol EDTA, RuCl 3 and 1 mL of 10% HCHO, and then placed in an infrared drying oven at 180 °C for 10 h. After cooling to room temperature, the sample was taken out and dried at 100 °C for 10 min to obtain a three-dimensional network nano-PdRu electrode.
[0026] The surface morphology of the prepared electrode was measured by a JSM6380LV scanning electron microscope ( figure 1 b) and energy spectrum analysis ( figure 2 b). The electrochemical test was carried out on atoLABPGSTA30 / FRA, a three-chamber glass electrolytic cell, the working electrode was a three-dimensional network nano-PdRu electrode, the counter electrode was a large-area Pt electrode, and the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com