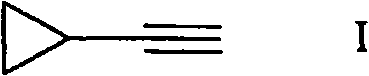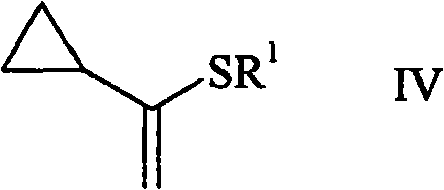Process for the synthesis of ethynylcyclopropane
A technology of ethynyl cyclopropane and alkyl group is applied in the synthesis field of ethynyl cyclopropane, which can solve the problems of low reaction temperature and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
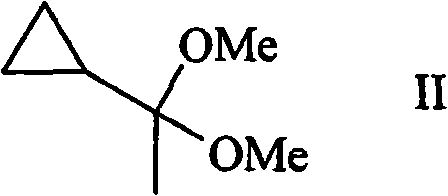
[0043] Example 1: (1,1-dimethoxy-ethyl)-cyclopropane (II)
[0044] At 25°C, add 82 grams (0.77 moles) of trimethyl orthoformate, 200 mL (4.94 moles) of methanol and 0.25 p-methylsulfonic acid to a flask containing 50 grams (0.59 moles) of cyclopropylmethyl ketone. g (1.45 mmol). The resulting solution was stirred at 25°C for 2 hours. 0.15 g (2.78 mmol) of sodium methoxide was added to the solution under vigorous stirring. Methyl formate, methanol and trimethyl orthoformate were removed under reduced pressure (approximately 250-300 mbar). The residue was washed with 100 ml of brine and dried over 15 g of sodium sulfate. Finally, a colorless liquid of (1,1-dimethoxyethyl)cyclopropane with a concentration of 45-65% is obtained.
Embodiment 2
[0045] Example 2: (1-Cyclopropylvinylsulfanyl)-Benzene (IV)
[0046] Add 1.3 grams (0.10 moles) of (1,1-dimethoxyethyl) cyclopropane, 11 grams (0.10 moles) of thiophenol, 250 milliliters of toluene and 700 grams (3 mmoles) of 10-camphorsulfonic acid In a flask with a funnel attached. The pressure-balanced dropping funnel was equipped with molecular sieves (beads) and a reflux condenser was installed at the top. The solution was refluxed at 130°C (bath temperature) for 20 hours. The mixture was washed with 10% aqueous NaOH (2 x 50 mL) and brine, and dried over 5 g of sodium sulfate. The toluene was removed under reduced pressure to yield an oil containing 46% (1-cyclopropylvinylsulfanyl)benzene. The crude product can be further purified, eg by distillation.
Embodiment 3
[0047] Embodiment 3: Ethynyl cyclopropane (I)
[0048] KAPA (potassium aminopropylamide) is either generated in situ from potassium hydride and 1,3-diaminopropane (Brown, C.A.J., Org. Chem. 1978.43.3083-3084), or in the presence of Fe(NO 3 ) 3 Generated in situ from potassium and 1,3-diaminopropane (Kimmel, T et al. Org. Chem. 1984, 49.2494-2496) in the presence of a catalyst. 358 mg (2.03 mmol) of (1-cyclopropylvinyl) sulfanylbenzene was added dropwise to a flask containing a newly generated KAPA solution (10 ml at 1,3 - in diaminopropane, concentration 1.27 mol / L, 12.7 mmol). The mixture was stirred at 25°C for 3 hours, then cooled to 0°C. Add ice water to the mixture. The yield of ethynylcyclopropane in the raw product was 88%. The mixture was washed with 2 x 50 mL of AcOH aqueous solution and 100 mL of brine, and washed with 2 g of Na 2 SO 4 dry. The raw product is purified by distillation. The boiling point is: 50-51°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com