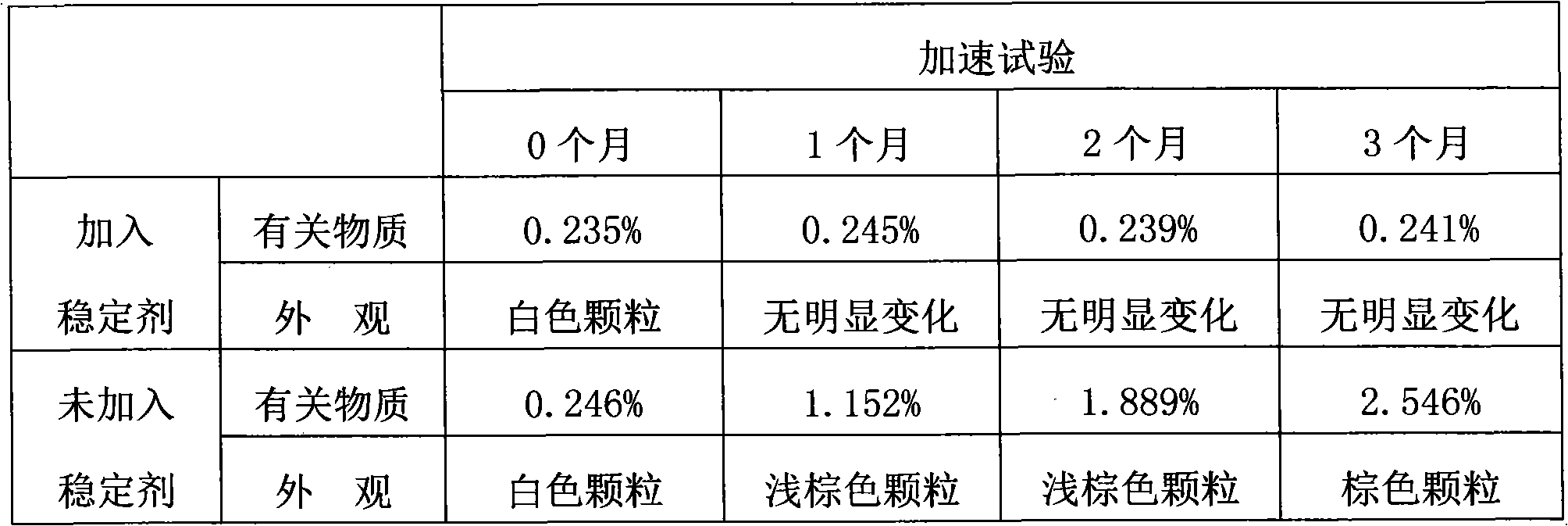
Preparation for promoting stability of ceftriaxone sodium granules
A ceftriaxone sodium and stabilizer technology, which is applied in the field of ceftriaxone sodium granules, can solve the problems of poor sensitivity and achieve the effects of simple operation, improved appearance and storage time, and cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0020] Ceftriaxone Sodium 0.3g
[0021] Glucose 3.5g
[0022] Mannitol 5.5g
[0023] Aspartame 0.2g
[0024] Sodium metabisulfite 0.005g
[0025] Flavor 0.05g
example 2
[0027] Ceftriaxone Sodium 0.3g
[0028] Lactose 4.5g
[0029] Sucrose 5.0g
[0030] Aspartame 0.1g
[0031] Thiourea 0.01g
[0032] Flavor 0.1g
example 3
[0034] Ceftriaxone Sodium 0.5g
[0035] Glucose 1.7g
[0036] Mannitol 4.6g
[0037] Aspartame 0.1g
[0038] Sodium bisulfite (potassium) 0.03g
[0039] Flavor 0.05g
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com