Method for preparing complete methanation catalyst for hydrothermal chemical process
A complete methanation, chemical process technology, applied in the field of synthesis gas complete methanation catalyst preparation, to achieve the effects of enhanced energy/resource security, low cost, good catalytic activity and hydrothermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: Catalyst A
[0048] (1) Weigh 130g Ni(NO 3 ) 2 .6H 2 O, 341g Al(NO 3 ) 3 .9H 2 O, add deionized water to dissolve to 1000mL;
[0049] (2) Weigh 160g of urea, add 500mL of deionized water to dissolve;
[0050] (3) After mixing the solutions (1) and (2), add deionized water to 2000mL,
[0051] (4) Heating the solution to 120°C and keeping the reaction for 8 hours;
[0052] (5) Filter the precipitate, wash twice with 1000mL deionized water; dry at 110°C for 12 hours; place the sample in a muffle furnace after drying, and roast at 500°C for 2 hours at a heating rate of 1°C / min; the resulting product is added with 3% Graphite and 2% cellulose were molded, and then calcined in a muffle furnace at 500°C for 2 hours to form a catalyst precursor.
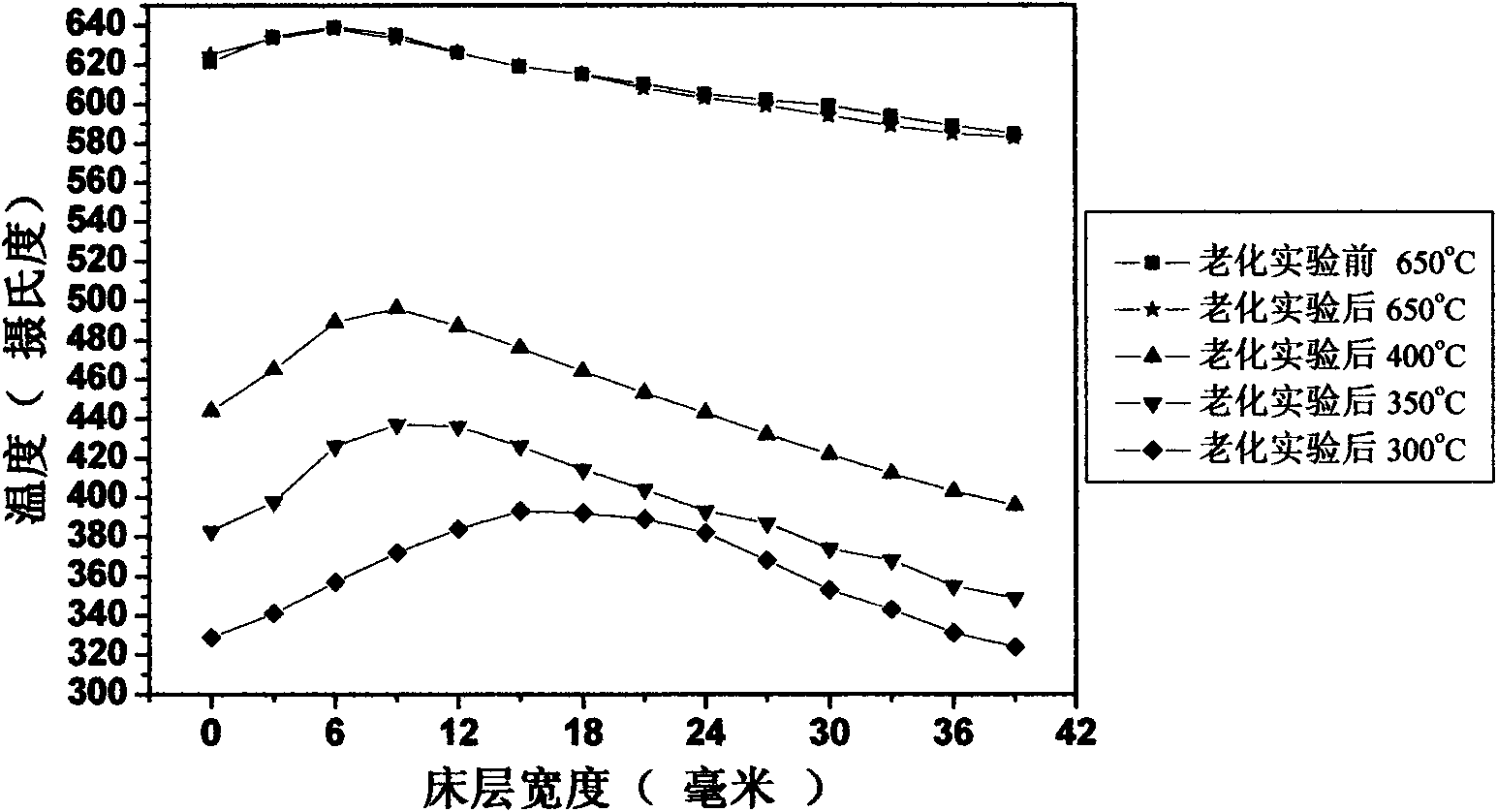
[0053] Gained catalyst precursor total 80g, composition is 42%NiO-58%Al 2 o 3 ; Under 500 DEG C of hydrogen conditions, it was reduced for 4 hours to obtain catalyst A, which was evaluated according to the met...
Embodiment 2
[0054] Example 2: Catalyst B
[0055] (1) Weigh 130g Ni(NO 3 ) 2 .6H 2 O, 253g Al(NO 3 ) 3 .9H 2 O, 1M Zr(NO 3 ) 4 Solution 97.5mL, add deionized water to dissolve to 1000mL;
[0056] (2) Weigh 150g of urea, add 500mL of deionized water to dissolve;
[0057] (3) After mixing the solutions (1) and (2), add deionized water to 2000mL,
[0058] (4) Heating the solution to 120°C and keeping the reaction for 8 hours;
[0059] (5) Filter the precipitate, wash twice with 1000mL deionized water; dry at 110°C for 12 hours; place the sample in a muffle furnace after drying, and roast at 500°C for 2 hours at a heating rate of 1°C / min; the resulting product is added with 3% Graphite and 2% cellulose were molded, and then calcined in a muffle furnace at 500°C for 2 hours to form a catalyst precursor.
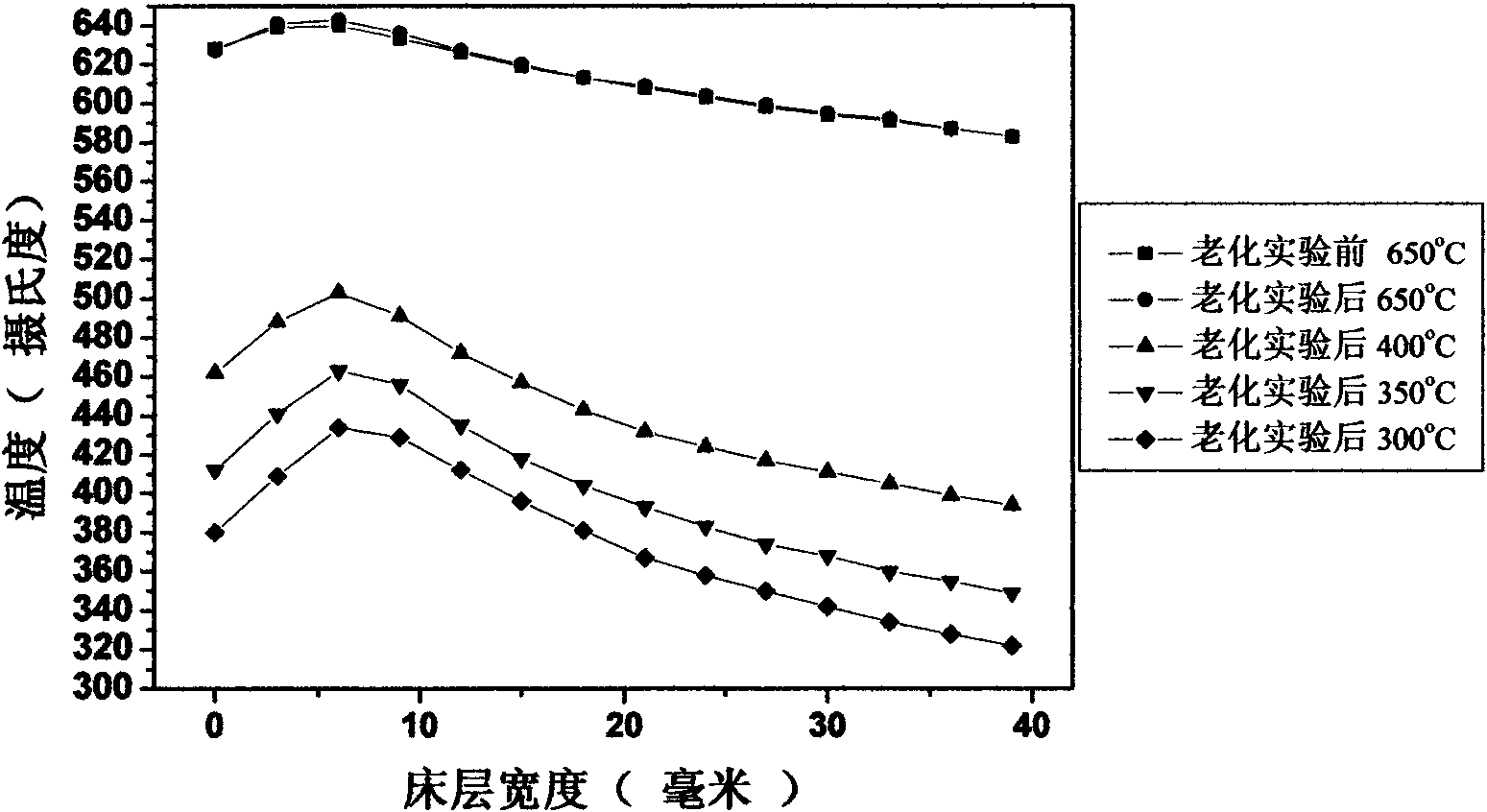
[0060] Gained catalyst precursor total 80g, composition is 42%NiO-43%Al 2 o 3 -15% ZrO 2 ; Under 500 DEG C of hydrogen conditions, it was reduced for 4 hours to obtain catalyst ...
Embodiment 3
[0061] Example 3: Catalyst C
[0062] (1) Weigh 130gNi(NO 3 ) 2 .6H 2 O, 235gAl(NO 3 ) 3 .9H 2 O, 6.4gLa(NO 3 ) 3 .6H 2 O, 1MZr(NO 3 ) 4 Solution 97.5mL, add deionized water to dissolve to 1000mL;
[0063] (2) Weigh 145g of urea, add 500mL of deionized water to dissolve;
[0064] (3) After mixing the solutions (1) and (2), add deionized water to 2000mL,
[0065] (4) Heating the solution to 120°C and keeping the reaction for 8 hours;
[0066] (5) Filter the precipitate, wash twice with 1000mL deionized water; dry at 110°C for 12 hours; place the sample in a muffle furnace after drying, and roast at 500°C for 2 hours at a heating rate of 1°C / min; the resulting product is added with 3% Graphite and 2% cellulose were molded, and then calcined in a muffle furnace at 500°C for 2 hours to form a catalyst precursor.
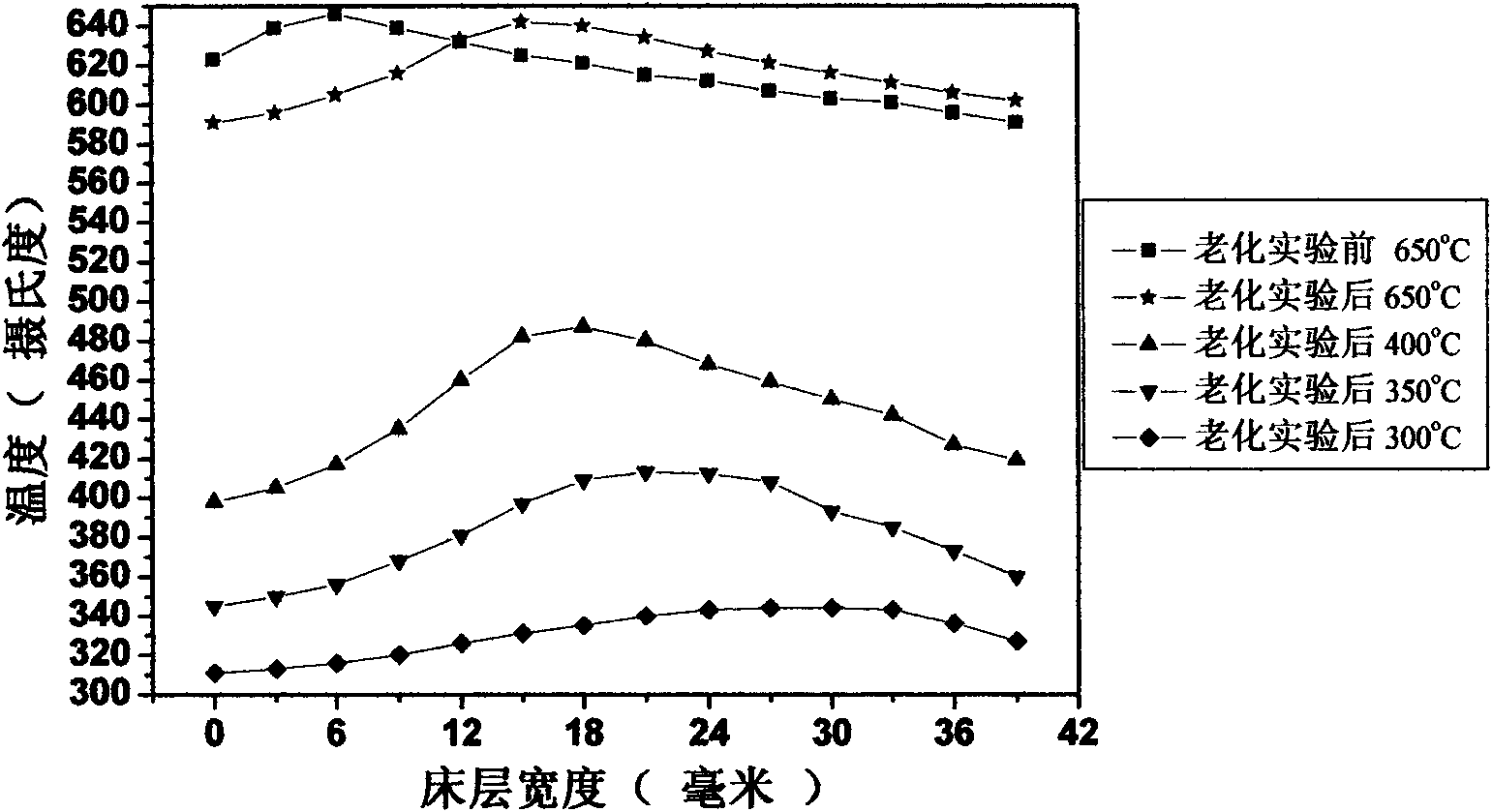
[0067] The resulting catalyst precursor is 80g in total, and the composition is 42%NiO-40%Al 2 o 3 -15% ZrO 2 -3% La 2 o 3 Reduction under 500 DEG C o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com