Thienopyridine derivative, and preparation method and application thereof
A derivative, thiophene technology, applied in the field of medicine, can solve the problems of high mortality and poor prognosis of cancer patients, achieve the effect of inhibiting proliferation, increasing yield, and avoiding the operation of filtering and washing enaminone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
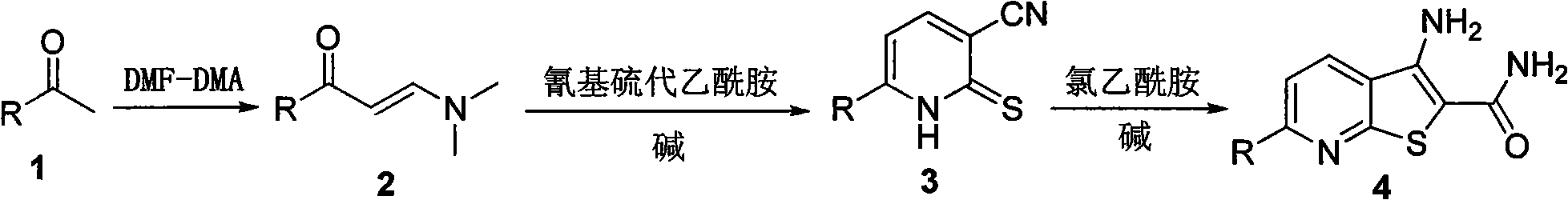
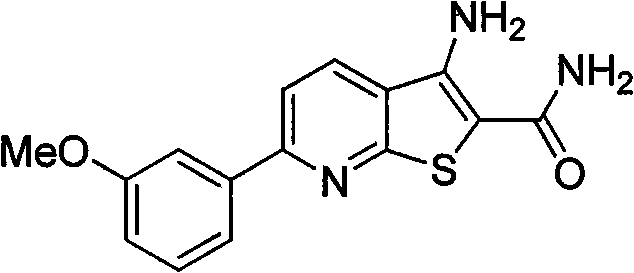
[0010] Example 1 Preparation of 3-amino-6-(3-methoxyphenyl)thiophene[2,3-b]pyridine-2-carboxamide (4a)
[0011]
[0012] Heat m-methoxyacetophenone (5g, 33.3mmol) and N,N-dimethylformamide dimethyl acetal (DMFDMA) (9.2ml, 69.3mmol) to reflux until the reaction of the raw materials is complete, then cool the reaction in an ice bath liquid, the solid was separated out by suction filtration, the filter cake was washed with cold petroleum ether and cold ethanol, and dried to obtain 5.2 g of a yellow solid with a yield of 75%, which was directly used in the next reaction.
[0013] The above enaminone solid (1g, 4.9mmol), 2-cyanothioacetamide (0.74g, 7.35mmol), 1,4-diazabicyclo[2.2.2]octane (DABCO) (0.54 g, 2.45mmol) was dissolved in 8ml of absolute ethanol, heated to reflux until the reaction was complete, cooled the reaction solution to room temperature, adjusted the reaction system to neutrality with dilute hydrochloric acid, filtered the solid out by suction filtration, washe...
Embodiment 2
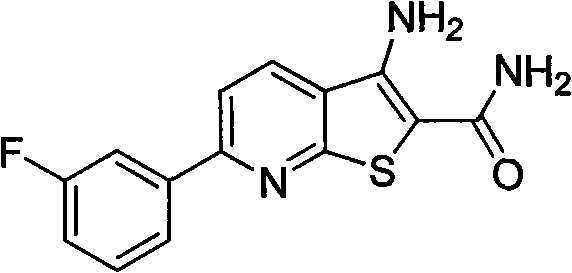
[0018] Example 2 Preparation of 3-amino-6-(3-fluorophenyl)thiophene[2,3-b]pyridine-2-carboxamide (4b)
[0019]
[0020] Heated m-fluoroacetophenone (5g, 36.2mmol) and N,N-dimethylformamide dimethyl acetal (DMF DMA) (10ml, 73.1mmol) to reflux until the reaction was complete, cooled the reaction solution in an ice bath, and pumped The solid was filtered out, and the filter cake was washed with cold petroleum ether and cold ethanol to obtain 4.8 g of a yellow solid with a yield of 69%, which was directly used in the next reaction.
[0021] The above-mentioned enaminone solid (1g, 5.2mmol), 2-cyanothioacetamide (0.8g, 7.7mmol), 1,4-diazabicyclo[2.2.2]octane (DABCO) (0.6 g, 2.6mmol) was dissolved in 8ml of absolute ethanol, heated to reflux until the reaction was complete, cooled the reaction solution to room temperature, adjusted the reaction system to neutrality with dilute hydrochloric acid, filtered the solid out by suction filtration, washed the filter cake with absolute et...
Embodiment 3
[0028] Example 3 Preparation of 3-amino-6-(3,4-dichlorophenyl)thiophene[2,3-b]pyridine-2-carboxamide (4c)
[0029]
[0030] 3,4-Dichloroacetophenone (5g, 26.5mmol) and N,N-dimethylformamide dimethyl acetal (DMFDMA) (8ml, 53.53mmol) were heated to reflux until the reaction was complete, and the reaction was cooled in an ice bath Liquid, the solid was separated out by suction filtration, the filter cake was washed with cold petroleum ether and cold ethanol, and dried to obtain 5.7 g of brown-red solid with a yield of 88%, which was directly used in the next reaction.
[0031] The above-mentioned enaminone solid (1g, 4.1mmol), 2-cyanothioacetamide (0.6g, 6.2mmol), 1,4-diazabicyclo[2.2.2]octane (DABCO) (0.5 g, 2mmol) was dissolved in 8ml of absolute ethanol, heated to reflux until the reaction was complete, cooled the reaction solution to room temperature, adjusted the reaction system to neutrality with dilute hydrochloric acid, filtered the solid out by suction filtration, was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com