Chiral spiro-phosphate and preparation method and application thereof
A spirocyclic phosphoric acid and chiral technology, which is applied in the field of chiral spirocyclic phosphoric acid and its preparation and application, can solve the problems of limited types of chiral phosphoric acid catalysts, and achieve good activity and enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
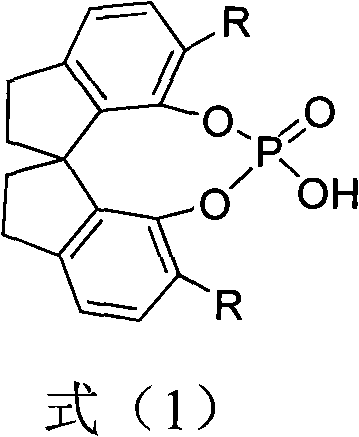
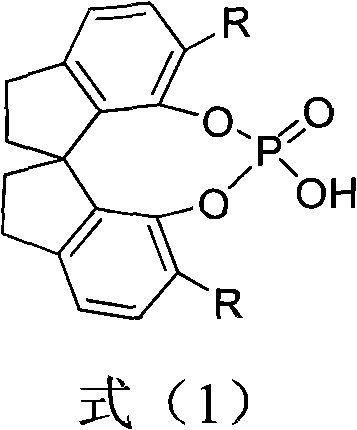
[0028] Example 1 Preparation of (S)-O,O'-{7,7'-[6,6'-bis-(1-naphthyl)-1,1'-spirodihydroindene]} phosphoric acid The structural formula is as follows :
[0029]
[0030] In the first step, 10 millimoles of (S)-1,1'-spiroindan-7,7'-diphenol and 30 millimoles of sodium hydride (60% content stored in mineral oil) are mixed in 100 ml In tetrahydrofuran, then 25 millimoles of chloromethyl methyl ether was added dropwise to the above reaction solution within 30 minutes at a temperature of 0°C and reacted at room temperature for 12 hours after the drop was completed. After the reaction was completed, 200 ml of water and 500 ml were added. Ethyl acetate, the organic phase was washed with 500 ml of saturated brine, dried over anhydrous sodium sulfate, filtered to remove sodium sulfate, and the filtrate was concentrated under reduced pressure to dryness to obtain a quantitative compound C;
[0031] In the second step, under the protection of nitrogen, mix 10 millimoles of compound C and 20 m...
Embodiment 2
[0034] Example 2 Preparation of various chiral spirocyclic phosphates
[0035] The preparation process is the same as in Example 1, but 1-naphthylboronic acid is replaced by phenylboronic acid, p-chlorophenylboronic acid, biphenylboronic acid or 3,5-ditrifluoromethylphenylboronic acid. The physical data of the corresponding chiral spirocyclic phosphate are as follows:
[0036] (S)-O,O’-{7,7’-[6,6’-diphenyl-1,1’-spirodihydroindene]} phosphoric acid
[0037]
[0038] The yield was 88%.
[0039] Melting point is greater than 300℃; [α] D 20 = -513.2 (c = 0.50, MeOH). 1 H NMR(400MHz, DMSO-d 6 )δ1.88-1.95(m, 2H), 2.18-2.22(m, 2H), 2.75-2.81(m, 2H), 3.01-3.10(m, 2H), 7.01(d, J=7.2Hz, 2H) , 7.10 (d, J = 7.6 Hz, 2H), 7.19 (t, J = 7.2 Hz, 2H), 7.28 (t, J = 7.6 Hz, 4H), 7.59 (d, J = 7.6 Hz, 4H); 13 C NMR(100MHz, DMSO-d 6 )δ30.0, 39.0, 59.5, 120.1, 126.3, 127.9, 129.7, 130.0, 134.6, 140.2, 142.0, 144.2, 146.3; 31 P NMR(202MHz, DMSO-d 6 )δ-11.0; HRMS(ESI)calcd for C 29 H 22 O 4 P - ([M-H] - ): 46...
Embodiment 3
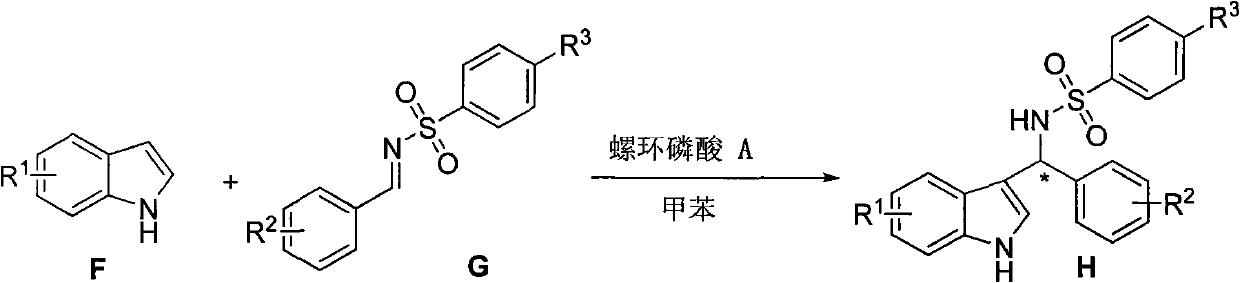
[0052] Example 3 Chiral spirocyclic phosphoric acid catalyzes the reaction of indole compounds with sulfonimide derivatives
[0053] Combine 1 mmol sulfonimide derivative G, 5 mmol indole derivative F, and 0.1 mmol (S)-O,O'-{7,7'-[6,6'-bis-(1- Naphthyl)-1,1'-spirodihydroindene]} phosphoric acid was mixed in 5 ml of toluene solvent, reacted at -60°C for a certain period of time (see Table 1), and then sodium hydroxide solution was added to neutralize the reaction. It was extracted with ethyl acetate, washed with saturated brine, dried over sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure, and then the chiral 3-alkylated indole derivative H was obtained by column chromatography. The optical purity of the product was determined by HPLC. The results are shown in Table 1.
[0054]
[0055] The structural formula of the catalyst spirocyclic phosphoric acid A here is:
[0056]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com