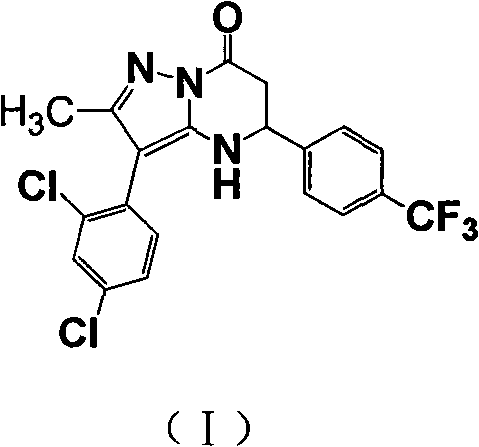
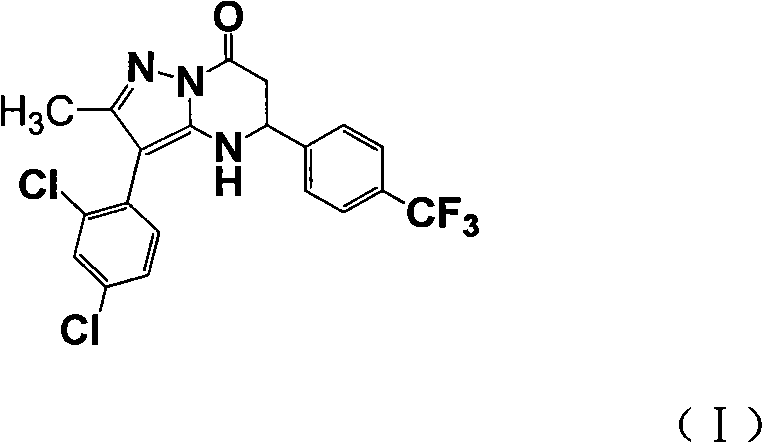
Medicament combination preparation of medicinal compound containing cy-ethyl razepam
A technology of composition and complex, which is applied in the direction of drug combination, medical preparations containing active ingredients, drug delivery, etc., and can solve problems such as increased solubility, narrow therapeutic window, and poor safety in clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1, cyclopyriminapine maleate injection
[0028] 【prescription】
[0029] Cyclopyrene Maleate 125g
[0030] Glucose 50g
[0031] Add water for injection to 1000ml
[0032] 【Method】
[0033] ①Dissolve glucose powder in water for injection to make a solution of about 50%~60%, adjust the pH value to about 4.2~4.6 with 10% HCl, heat and boil for 10~20min, add 0.05% activated carbon, stir well, and cool to room temperature, filter out activated carbon, measure the content, and set aside.
[0034] ②Take the dosing amount of glucose (calculated as volume according to the content), add cyclopyridine maleate, heat to 40-50°C, stir to dissolve, dilute to the dosing amount, cool to room temperature, and measure the pH value at 4.2-4.8 Finely filter until clear, fill and seal in 2ml ampoules, and sterilize with circulating steam at 100°C for 30 minutes.
Embodiment 2
[0035] Embodiment 2, cyclopyriminapine maleate injection
[0036] This product is a sterilized aqueous solution of cordocaine hydrochloride. Cyclopyriminapine containing maleate should be 95% to 105% of the labeled amount.
[0037] 【prescription】
[0038] Cyclopyrene Maleate 125g
[0040] Add water for injection to 1000ml
[0041] [Preparation method] Dissolve sodium chloride in water for injection, add cyclopyrimidine maleate and stir to dissolve, dilute to the full amount, adjust the pH to 3.5-5.0 with dilute hydrochloric acid, add activated carbon, filter, potting, 100°C Circulating steam sterilization for 3 minutes, that is.
Embodiment 3
[0042] Embodiment 3, Cyclopyrimine Injection
[0043] 【prescription】
[0044] Cyclopyrene 1250g Glycerin 500g
[0045] Ethanol (95%) 1000ml water for injection to 10000ml
[0046] [Preparation method] Dilute ethanol with water for injection to about 70%, add lanatoside triacetate, stir continuously to dissolve, add glycerin, dilute with water for injection to the total amount, filter until clear, and seal in a 2ml ampoule. Sterilize with flowing steam at 100°C for 30 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com