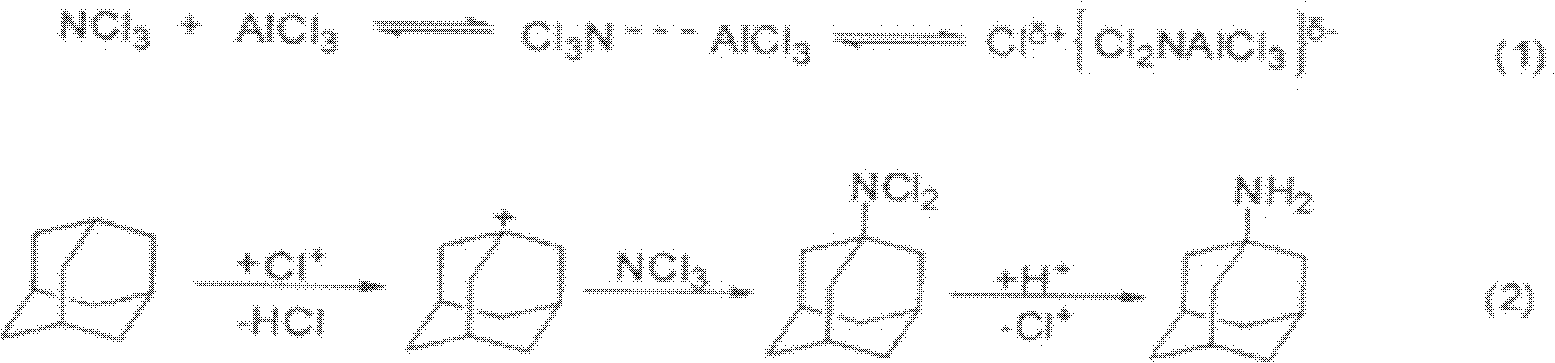Synthesis method for Amantadine Hydrochloride
A technology of amantadine hydrochloride and its synthesis method, which is applied in the field of pharmaceuticals, can solve the problems of strong corrosion, expensive bromine, and difficult recovery, and achieve the effects of promoting isomerization reaction, increasing yield, and avoiding the generation of tar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] Example. The synthetic method of amantadine hydrochloride comprises the following steps, 1), hydrogenation: under the catalysis of nickel catalyst, dicyclopentadiene, hydrogenation reaction, control reaction pressure 1.5~1.8MPa, reaction temperature 110~155 ℃, early stage The temperature is 110-140°C. When the reaction does not absorb hydrogen, continue to heat and hold the pressure for 8 hours to obtain tetrahydrodicyclopentadiene; 2), isomerization and amination: according to the following weight ratio: tetrahydrodimerization 60 grams of cyclopentadiene, 20 grams of aluminum trichloride, and 120 milliliters of trichloroethane were reacted for 30 minutes at 30° C., wherein aluminum trichloride was the main catalyst and trichloroethane was the solvent, and then the temperature was slowly raised to 80 ℃, keep warm for 5 hours; when the temperature drops to about -10℃, start to add 10% nitrogen trichloride-trichloroethane solution dropwise, and keep the dropping temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com