Method for preparing alpha-glucosylhesperidin
A technology of glucosyl and hesperidin, which is applied in the field of preparation of modified hesperidin, can solve the problems that other functions of hesperidin have not been evaluated, and achieve the effects of high yield, mild reaction conditions and simple route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
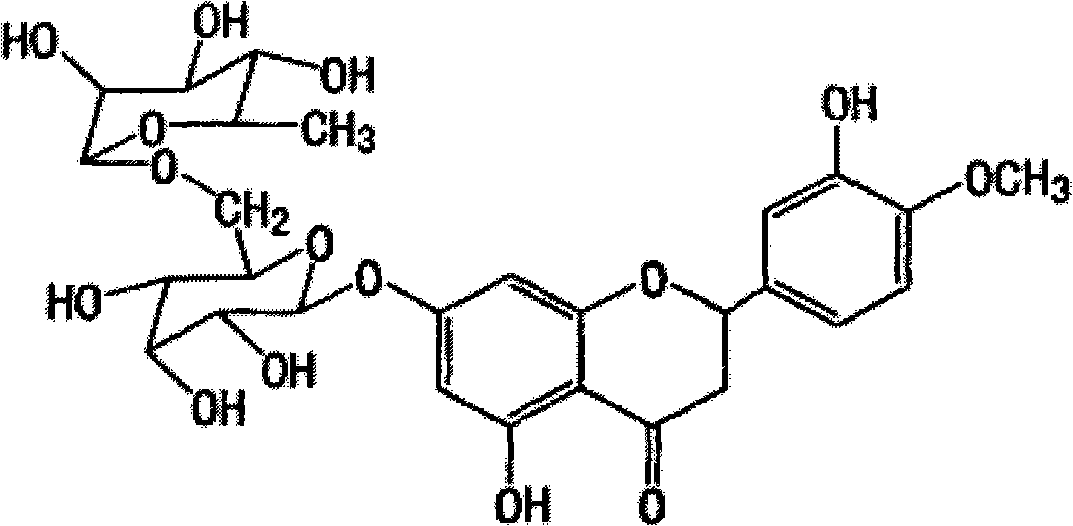
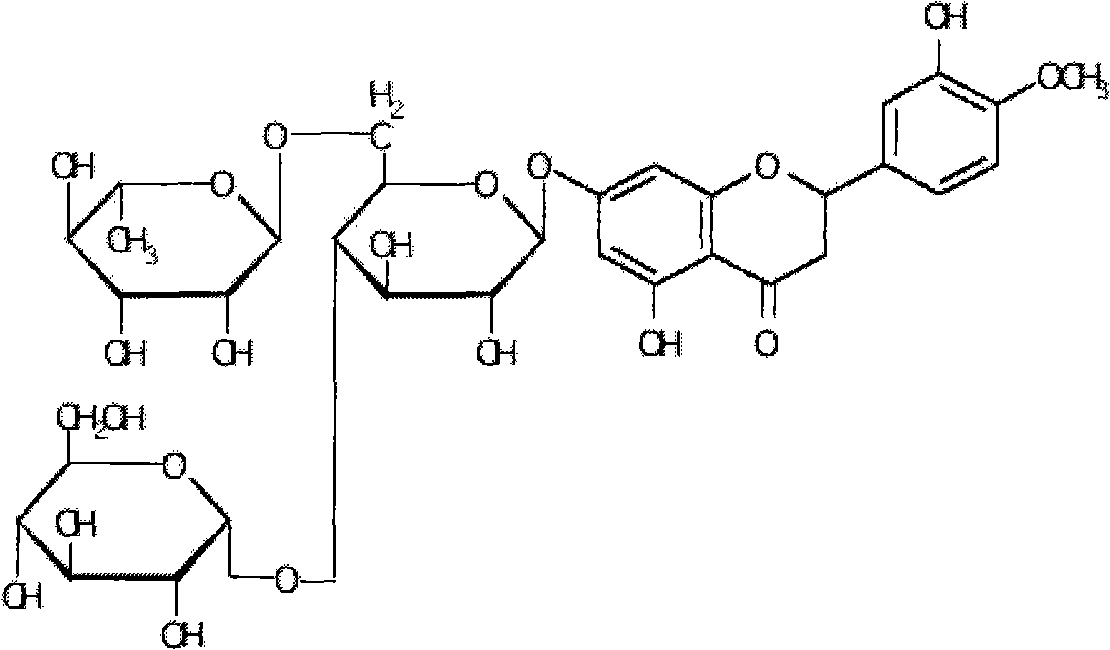
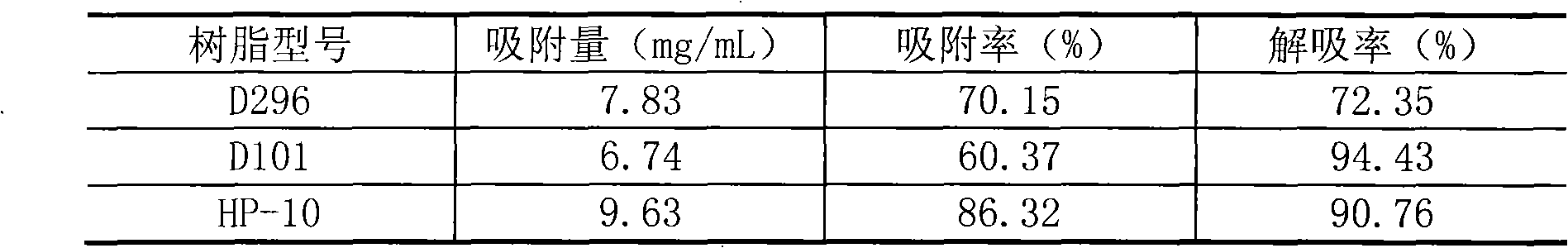
[0037] Example 1: Mix 5.0 g of hesperidin with 20 ml of 5% dextrin, then add 2.5 ml of α-glucosyltransferase, adjust the pH value to about 6.0, and react at 55° C. for 24 hours. HPLC detection showed that hesperidin was partially transformed into α-glucosyl hesperidin. After the reaction was completed, the reaction mixture was heated to 80°C to inactivate the enzyme (referring to α-glucosyltransferase, the same below), and after standing overnight, it was filtered to obtain the supernatant. The supernatant is passed through HP-10 macroporous resin column, and then decolorized by activated carbon filtration (washing the column with distilled water to remove sugar and inorganic salts, and then washing the column with 70% ethanol solution), concentrated and crystallized to obtain the product α-glucosyl Hesperidin, the conversion rate of hesperidin in this process is 52.54%.
Embodiment 2
[0038] Example 2: Mix 5.0 g of hesperidin with 20 ml of 5% dextrin, add 3.0 ml of α-glucosyltransferase, adjust the pH value to about 6.0, and react at 55° C. for 24 hours. HPLC detection showed that hesperidin was partially transformed into α-glucosyl hesperidin. After the reaction, the reaction mixture was heated to 80°C to inactivate the enzyme, then the solvent (referring to water, the same below) was spin-dried, and then distilled water was added, and the supernatant was obtained by filtration after standing overnight. The supernatant was passed through a HP-10 macroporous resin column, then decolorized by activated carbon filtration (washing the column with distilled water to remove sugar and inorganic salts, and then washing the column with 70% ethanol solution), concentrated and spin-dried to obtain the product α-glucose Base hesperidin, the conversion rate of hesperidin in this process is 59.41%.
Embodiment 3
[0039] Example 3: Mix 5.0 g of hesperidin with 20 ml of 5% dextrin, add 3.0 ml of α-glucosyltransferase, adjust the pH value to about 7.0, and react at 60° C. for 24 hours. HPLC detection showed that hesperidin was partially transformed into α-glucosyl hesperidin. After the reaction, the reaction mixture was heated to 80°C to inactivate the enzyme, then the solvent was spin-dried and distilled water was added, and the supernatant was obtained by filtration after standing overnight. The supernatant is passed through HP-10 macroporous resin column, and then decolorized by activated carbon filtration (washing the column with distilled water to remove sugar and inorganic salts, and then washing the column with 70% ethanol solution), concentrated and crystallized to obtain the product α-glucosyl Hesperidin, the conversion rate of hesperidin in this process is 66.47%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com