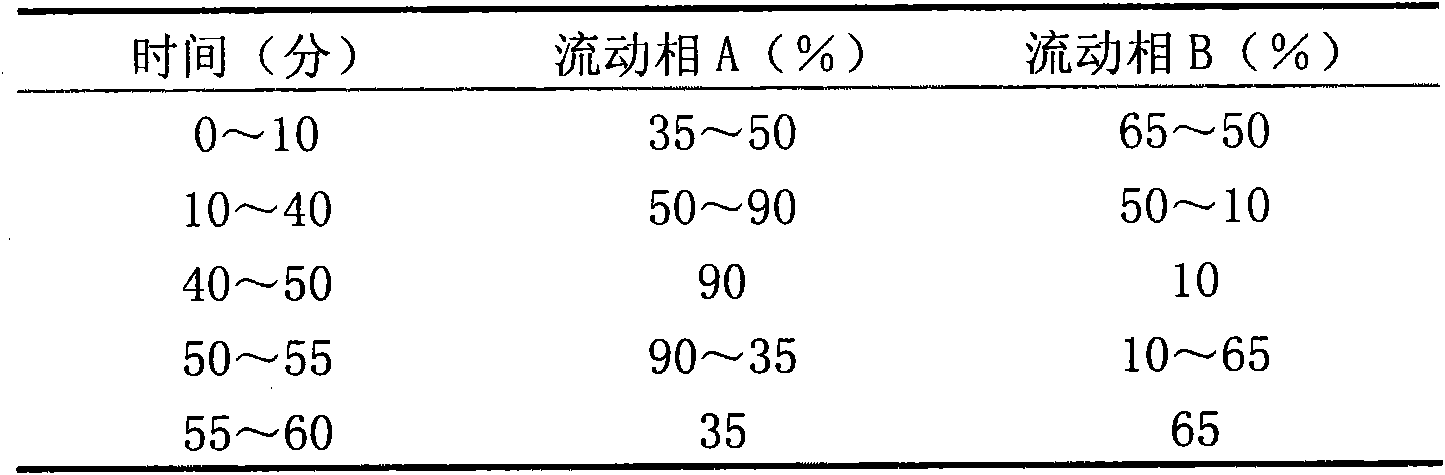
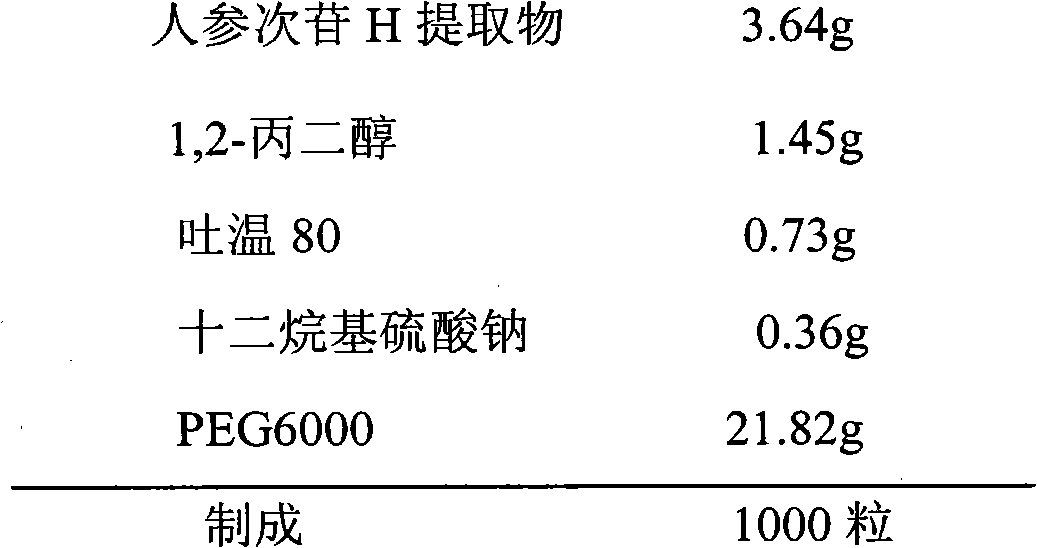
Ginseng saponin extract and preparation method thereof
A technology of ginsenosides and total ginsenosides is applied in the direction of testing pharmaceutical preparations, drug combinations, pharmaceutical formulations, etc., and can solve the problems of many by-products, difficult industrialized production, and low yield of active ingredients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1 Preparation of Ginsenoside H Extract
[0085] Step 1. Extract the American ginseng leaves with water twice for 2 hours each time. Add 10 times the amount of water each time. After being concentrated, the extract is passed through the D101 macroporous adsorption resin column, first eluted with water, and eluted until the effluent is nearly colorless, and then used Elute with 85% ethanol, collect the 85% ethanol eluate and concentrate to dryness to obtain total saponin;
[0086] Step 2. Dissolve 10kg of total saponins and 10kg of sodium hydroxide in 60kg of glycerin, heat, and control the reaction temperature at 230°C for a reaction time of 30min to obtain alkali degradation products.
[0087] Step 3. The alkali degradation product is heated with 95% ethanol to dissolve it, and then mixed with 2 times the amount of silica gel, dried and crushed and sieved to perform silica gel column chromatography. The ratio of alkali degradation product to chromatography silica gel i...
Embodiment 2
[0088] Example 2 Preparation of Ginsenoside H Extract
[0089] Step 1. Extract American ginseng leaves with water 3 times, 3 hours each time, add 10 times the amount of water each time, the extract passes through the D101 macroporous adsorption resin column, first eluted with water, eluted until the effluent is nearly colorless, and then used 50% Ethanol elution, collect the eluate, concentrate to dryness to obtain total saponin;
[0090] Step 2. Dissolve 10 kg of total saponins and 10 kg of potassium hydroxide in 60 kg of glycerol, heat, and control the reaction temperature at 150° C. and the reaction time for 60 minutes. Get alkaline degradation products,
[0091] Step 3. The alkali degradation product is heated with 85% ethanol to dissolve it, and 3 times the amount of silica gel is stirred, dried and crushed and sieved to perform silica gel column chromatography. The ratio of the alkali degradation product to the chromatography silica gel is 1:5. Elute with a solution system wi...
Embodiment 3
[0092] Example 3 Preparation of Ginsenoside H Extract
[0093] Step 1. The American ginseng is extracted with water, and the extract is passed through the AB-8 macroporous adsorption resin column, first eluted with water, and eluted until the effluent is nearly colorless, followed by elution with 95% ethanol, and collect the 95% ethanol eluate. Concentrate to dryness to obtain total saponins;
[0094] Step 2. Dissolve 10 kg of total saponins and 10 kg of sodium carbonate in 60 kg of glycerol, heat, and control the reaction temperature at 200° C. and the reaction time for 20 minutes. Get alkaline degradation products,
[0095] Step 3. Heat the alkali degradation product with 95% ethanol to dissolve it, stir in 2 times the amount of silica gel, dry and smash through a sieve, and perform silica gel column chromatography. The ratio of alkali degradation product to chromatography silica gel is 1:10. The volume ratio is chloroform-methanol (8:1) solution system for elution, thin-layer TL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com