Two-stage type nickel precipitation method
A two-stage, nickel-precipitating technology, applied in the direction of improving process efficiency, can solve the problems of harsh operating conditions, loss of precipitant, high equipment investment, etc., and achieve the effect of low production cost, fast precipitation speed and low investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
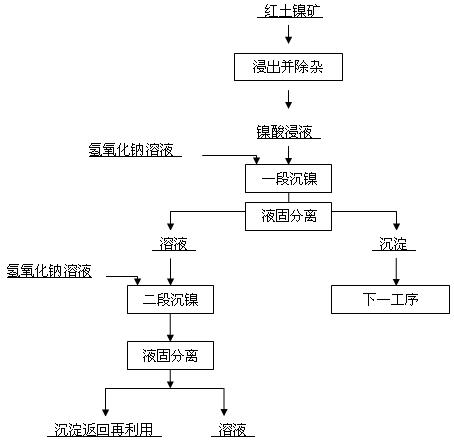
[0023] The lateritic nickel ore leach solution contains 3.43g / l nickel and 30.28 g / l magnesium. Add 40g / l sodium hydroxide aqueous solution, adjust the pH value to 7.5, the temperature is 80 degrees Celsius, react for 3 hours, and carry out liquid-solid separation. The obtained precipitate contains 0.12% magnesium and 50% nickel, and the separated solution contains 0.343g / l nickel and 30.26g / l magnesium. The solution obtained by separation continues to increase the pH value to 9.0 with 40 g / l aqueous sodium hydroxide solution, and the temperature is 80 degrees Celsius. After reacting for 3 hours, liquid-solid separation is carried out, and the precipitate contains 35.91% of nickel and 6.92% of magnesium. The separated solution contains Nickel 0.0099g / l, magnesium 30.20g / l.
Embodiment 2
[0025] The lateritic nickel ore leachate contains 2.9g / l nickel and 25.09g / l magnesium. Add 45g / l sodium hydroxide aqueous solution, adjust the pH value to 8.15, the temperature is 50 degrees Celsius, react for 1.5 hours, and carry out liquid-solid separation. The obtained precipitate contains 0.43% of magnesium and 47.45% of nickel, and the separated solution contains 0.29g / l of nickel and 25.07g / l of magnesium. The solution that separates obtains continues to raise pH value with the sodium hydroxide aqueous solution of 40g / l, and temperature is 50 degrees centigrade, reacts for 0.5 hour, carries out liquid-solid separation, obtains precipitation and contains nickel 36.5%, contains magnesium 7.48%, separates and obtains solution containing Nickel 0.0082g / l, magnesium 24.65g / l.
Embodiment 3
[0027] The laterite nickel ore leaching solution contains nickel concentration of 1.95g / l and magnesium content of 19.74 g / l. Add 50g / l sodium hydroxide aqueous solution, adjust the pH value to 8.5, the temperature is 20 degrees centigrade, react for 0.5 hour, and carry out liquid-solid separation. The obtained precipitate contains 0.95% of magnesium and 45.88% of nickel, and the separated solution contains 0.195g / l of nickel and 19.73g / l of magnesium. The solution obtained by separating continues to raise the pH value to 9.3 with the sodium hydroxide aqueous solution of 40g / l, 20 degrees Celsius of temperature, reacts 2 hours, carries out liquid-solid separation, obtains precipitation and contains nickel 38.1%, contains magnesium 7.62%, separates and obtains The solution contains 0.0076g / l nickel and 19.70g / l magnesium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com