Analgesic anti-inflammatory preparation for external application
An anti-inflammatory analgesic and topical technology, applied in anti-inflammatory, non-central analgesic, anesthetic and other directions, can solve the problems of unsatisfactory anti-inflammatory and analgesic effects, and achieve excellent transdermal absorption and ease skin irritation. , excellent permeability and diffusivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The preparation method of the external preparation provided by the present invention is not particularly limited. According to the desired dosage form, it can be prepared by fully kneading each component and the required matrix components, etc. for preparing an ordinary external preparation.
[0056] When preparing cataplasms and patches, it can be prepared by spreading the kneaded mixture on release paper, drying the kneaded mixture, bonding it to a soft support, and cutting it into a desired size.
[0057] The external preparations provided by the present invention, for example, when they are ointments, liquid preparations (suspensions, emulsions, lotions, etc.), aerosols, and external powders, are used according to the following usual methods of use: direct application by smearing, etc. Use on affected parts of the skin, or after applying or soaking on a support such as cloth.
[0058] In the case of a cataplasm or a patch, these preparations are directly attached to...
Embodiment 1
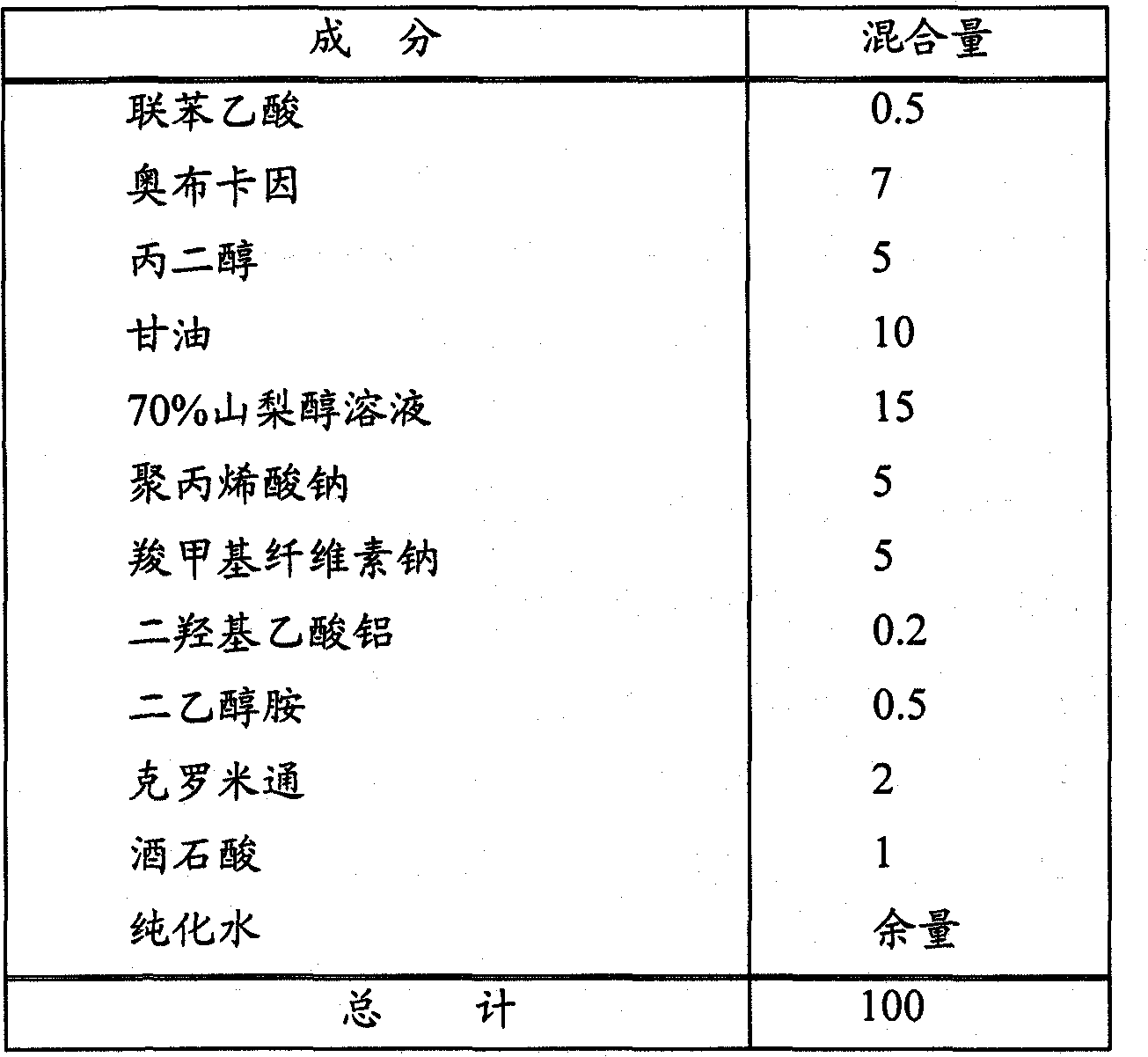
[0062] Drug-containing matrices of the formulations shown in Table 1 below were prepared. Specifically, felbinac was dissolved in crotamiton, oxybucaine was dissolved in propylene glycol, and the two were mixed, and then these dissolved substances and other ingredients shown in Table 1 were mixed until uniform to obtain Medicinal base. The drug-containing matrix prepared in this way was treated with 1000g / m 2 Spread on non-woven fabric, lined with polypropylene, then cut into 10×14cm 2 , to obtain an external patch.
[0063] [Table 1]
[0064]
[0065] Unit: parts by weight
Embodiment 2
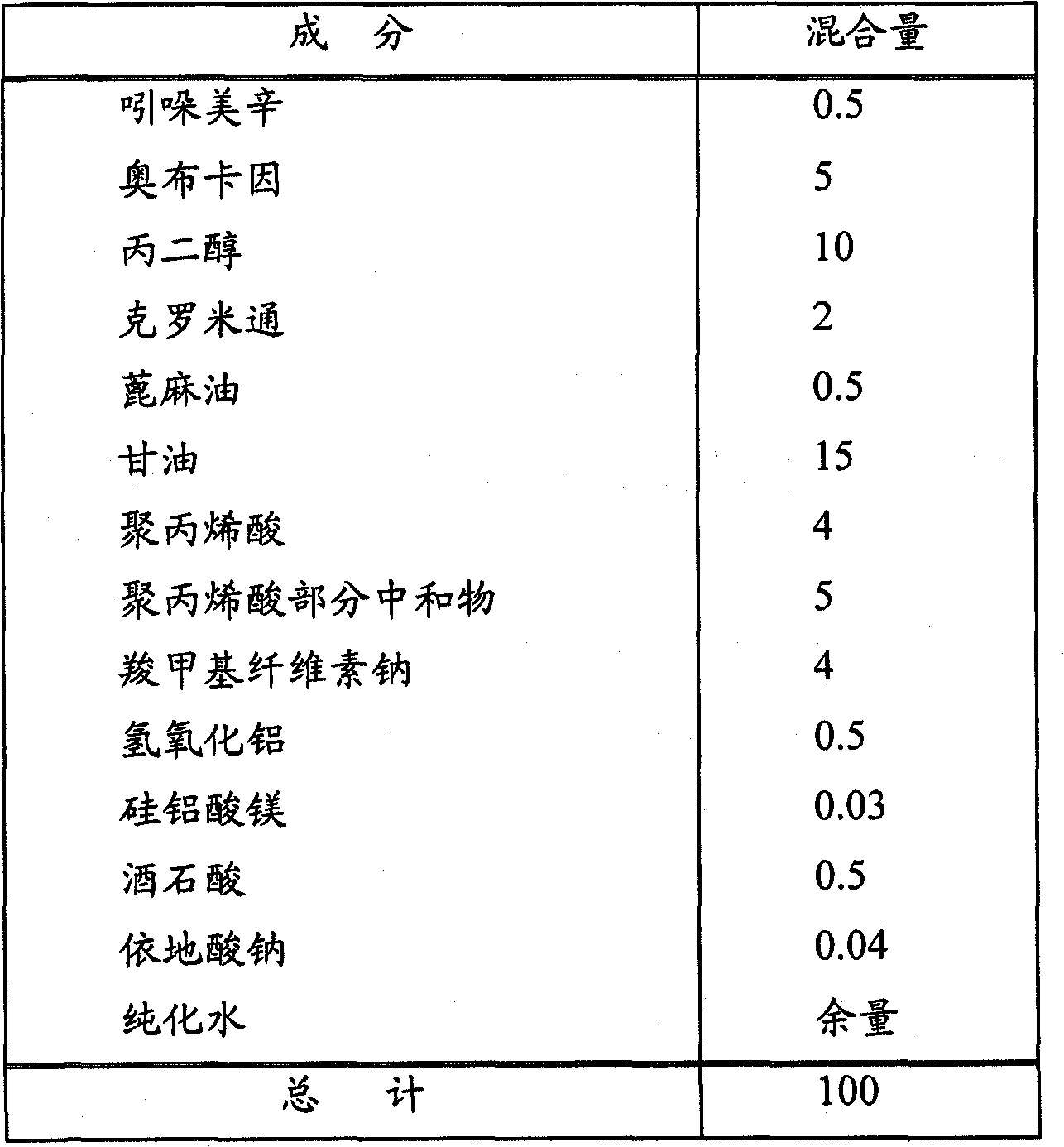
[0067] Drug-containing matrices of the formulations shown in Table 2 below were prepared. Specifically, indomethacin was dissolved in crotamiton and oxybucaine was dissolved in propylene glycol.
[0068] Then, these solubilized substances were mixed with other ingredients shown in Table 2 until uniform to obtain a drug-containing matrix. The drug-containing matrix prepared in this way was treated with 1000g / m 2 Spread on non-woven fabric, lined with polypropylene, then cut into 10×14cm 2 , to obtain an external patch.
[0069] [Table 2]
[0070]
[0071] Unit: parts by weight
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com