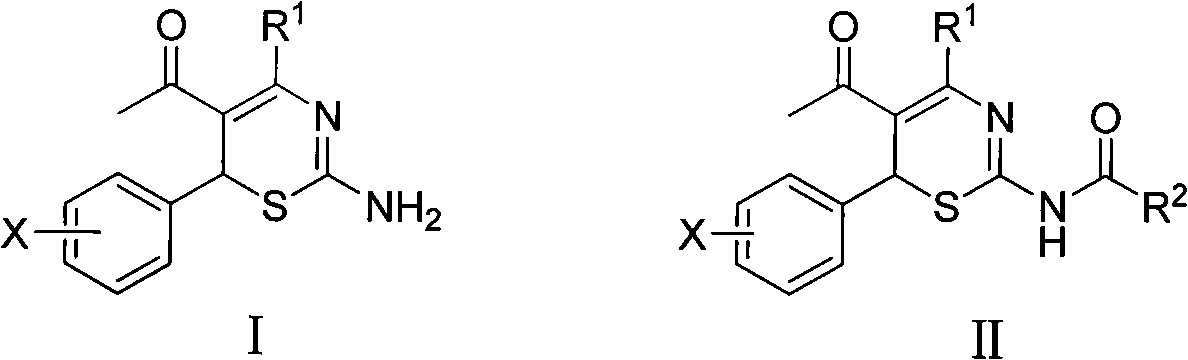
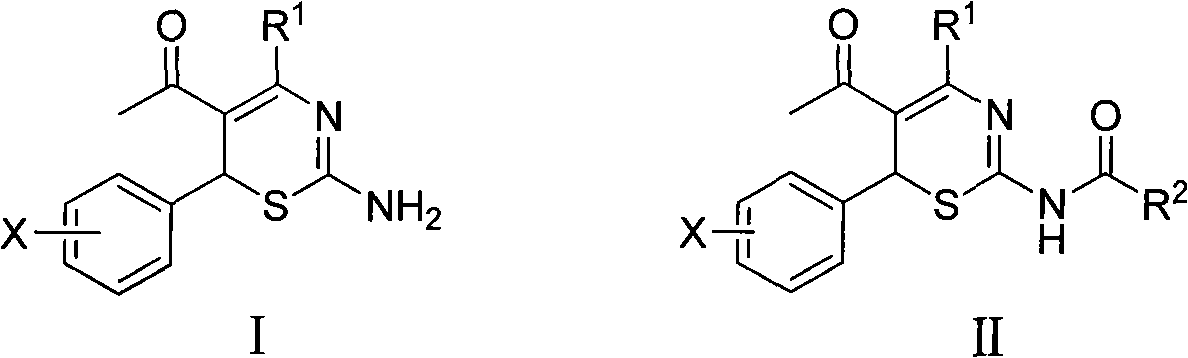
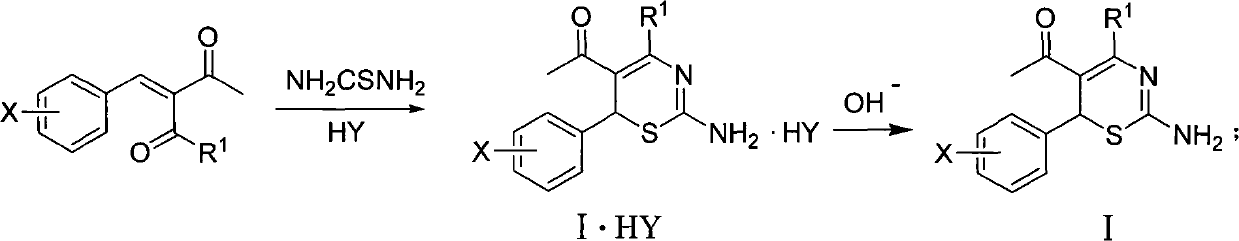
4-alkyl-6-aryl-5-acetyl-1, 3-thiazine, and preparation method and application thereof
A technology of alkyl and thiazine, which is applied in the fields of drug combination, active ingredient of heterocyclic compounds, organic chemistry, etc., and can solve the problem of no research and development reports on anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 4-methyl-6-(2-methoxyphenyl)-5-acetyl-2-amino-1,3-thiazine and 4-methyl-6-(2-methoxybenzene base)-5-acetyl-2-acetylamino-1, the preparation of 3-thiazine
[0023] (1) Preparation of 4-methyl-6-(2-methoxyphenyl)-5-acetyl-2-amino-1,3-thiazine [0023]8.5mmol thiourea, 8.5mmol 3-( 2-methoxybenzylidene)-2,4-pentanedione, 1ml of concentrated hydrochloric acid, 45ml of ethanol, reflux and water separation reaction for 6.0h, 4-methyl-6-(2-methoxybenzene base)-5-acetyl-2-amino-1,3-thiazine, the yield is 18%, and the melting point is 188-190°C. 1 H NMR (400M Hz, CDCl 3 ), δ: 2.20 (s, 3H, COCH 3 ), 2.49 (s, 3H, CH 3 ), 3.91 (s, 3H, OCH 3 ), 5.59 (s, 1H, thiazine ring 6-H), 6.83-7.24 (m, 4H, benzene ring).
[0024] (2) Preparation of 4-methyl-6-(2-methoxyphenyl)-5-acetyl-2-acetylamino-1,3-thiazine
[0025] 1.0mmol 4-methyl-6-(2-methoxyphenyl)-5-acetyl-2-amino-1,3-thiazine, 1.0ml acetic anhydride, appropriate amount of DMAP, 5.0ml tetrahydrofuran, at 45~60 Stir at ℃...
Embodiment 2
[0026] Example 2 4-methyl-6-(2-nitrophenyl)-5-acetyl-2-amino-1,3-thiazine and 4-methyl-6-(2-nitrophenyl) - Preparation of 5-acetyl-2-acetylamino-1,3-thiazine
[0027] (1) Preparation of 4-methyl-6-(2-nitrophenyl)-5-acetyl-2-amino-1,3-thiazine
[0028] 11mmol of thiourea, 11mmmol of 3-(2-nitrobenzylidene)-2,4-pentanedione, 1ml of concentrated hydrochloric acid, 50ml of ethanol, reflux and water separation for 6.5h, and 4-methyl-6 -(2-nitrophenyl)-5-acetyl-2-amino-1,3-thiazine, yield 47%, melting point 205-207°C.
[0029] (2) Preparation of 4-methyl-6-(2-nitrophenyl)-5-acetyl-2-acetylamino-1,3-thiazine
[0030] 2.0mmol 4-methyl-6-(2-nitrophenyl)-5-acetyl-2-amino-1,3-thiazine, 2.0ml acetic anhydride, appropriate amount of DMAP, 5.0ml tetrahydrofuran, at 45~60℃ Stir for 0.6h, spin evaporate the solvent, add water, filter with suction and dry to obtain 4-methyl-6-(2-nitrophenyl)-5-acetyl-2-acetylamino-1,3-thiazine. The yield is 63%, and the melting point is 166-168°C. 1 H NMR ...
Embodiment 3
[0031] Example 3 4-methyl-6-(3-nitrophenyl)-5-acetyl-2-amino-1,3-thiazine and 4-methyl-6-(3-nitrophenyl) - Preparation of 5-acetyl-2-acetylamino-1,3-thiazine
[0032] (1) Preparation of 4-methyl-6-(3-nitrophenyl)-5-acetyl-2-amino-1,3-thiazine
[0033] 10mmol of thiourea, 10mmmol of 3-(3-nitrobenzylidene)-2,4-pentanedione, 1ml of concentrated hydrochloric acid, 50ml of ethanol, reflux and water separation for 8.0h, and 4-methyl-6 -(3-nitrophenyl)-5-acetyl-2-amino-1,3-thiazine, yield 58%, melting point 177-178°C.
[0034] (2) Preparation of 4-methyl-6-(3-nitrophenyl)-5-acetyl-2-acetylamino-1,3-thiazine
[0035] 2.0mmol 4-methyl-6-(3-nitrophenyl)-5-acetyl-2-amino-1,3-thiazine, 2.0ml acetic anhydride, appropriate amount of DMAP, 5.0ml tetrahydrofuran, at 45~60℃ Stir for 1.1h, spin evaporate the solvent, add water, filter with suction and dry to obtain 4-methyl-6-(3-nitrophenyl)-5-acetyl-2-acetylamino-1,3-thiazine. The yield is 88%, and the melting point is 170-171°C. 1 H NMR (4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com