Preparation method of modified porous plate
A porous plate and modification technology, which is applied in the field of medical device modification and nanomaterials, can solve the problems of complicated experimental process, complicated operation and high cost, and achieve the effect of simple process, low cost and easy process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) 0.3g potassium bicarbonate (molecular weight 100), 0.03g glucose (molecular weight 198), 30mg chloroauric acid powder were dissolved in 6mL deionized water, and the pH value was adjusted to 9.0 with sodium hydroxide solution.
[0028] (2) Pipette 100 μL of the working solution into the microplate, and then place the microplate in an oven at 25° C. for 6 hours. The reaction solution in the wells was discarded, and then rinsed with deionized water for 3 times to prepare an ELISA plate modified with gold nanoparticles aggregates.
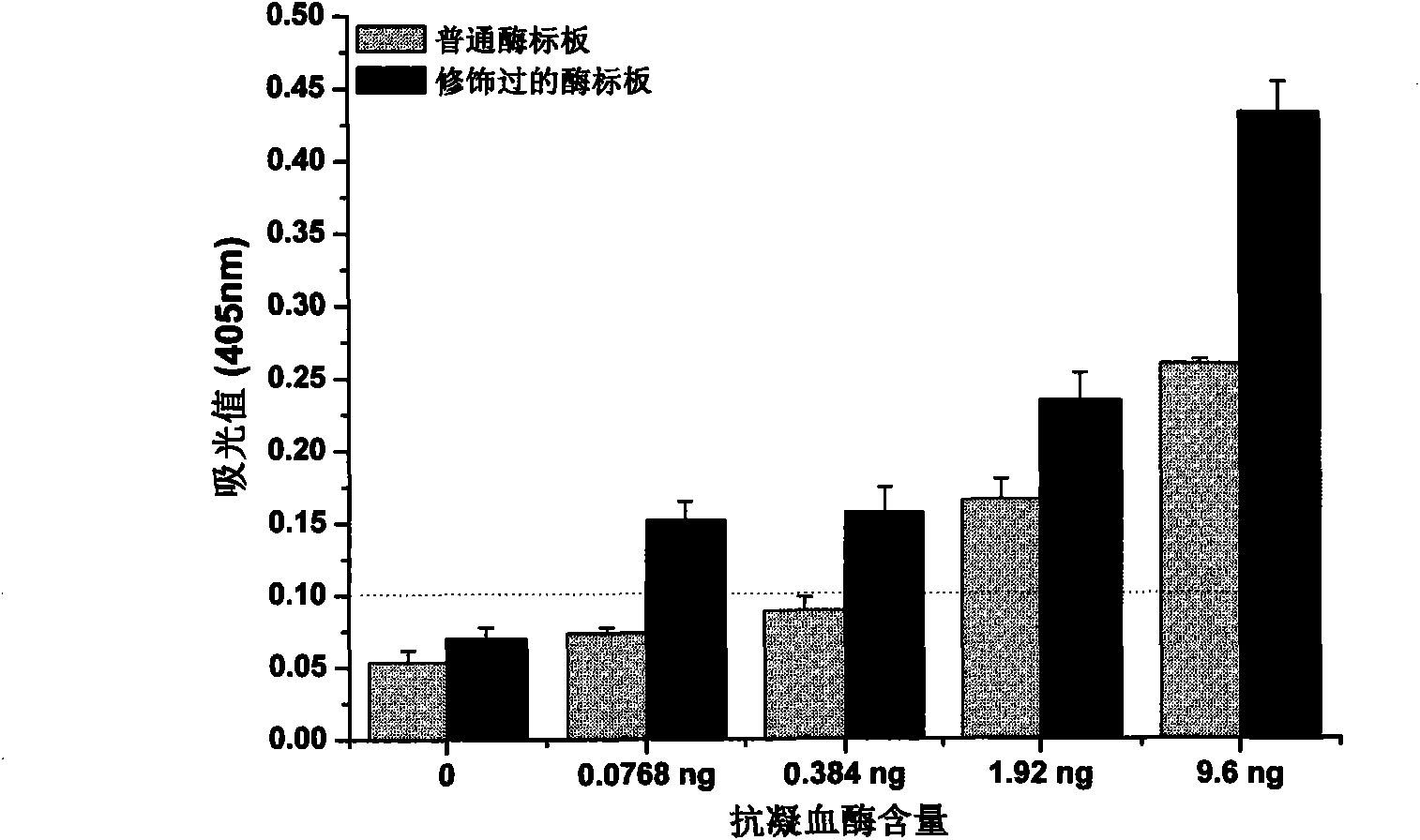
[0029] (3) Dilute human antithrombin with carbonate buffer (pH=9.6) to obtain protein solutions with concentrations of 96ng / mL, 19.2g / mL, 3.84ng / mL, and 0.768ng / mL . The above-mentioned gradient solution was coated on the ordinary microplate and the modified microplate at the dosage of 100 μL per well, and incubated in an oven at 37°C for 2 hours. Discard the coating solution in the plate, wash it with plate washing solution for 3 times, t...
Embodiment 2
[0032] (1) 0.3g potassium bicarbonate (molecular weight 100), 0.03g glucose (molecular weight 198), 30mg chloroauric acid powder were dissolved in 6mL deionized water, and the pH value was adjusted to 9.0 with sodium hydroxide solution.
[0033] (2) Pipette 250 μL of the working solution into the microplate, and then place the microplate in an oven at 50° C. for 1 hour to react. The reaction solution in the wells was discarded, and then rinsed with deionized water for 3 times to prepare an ELISA plate modified with gold nanoparticles aggregates.
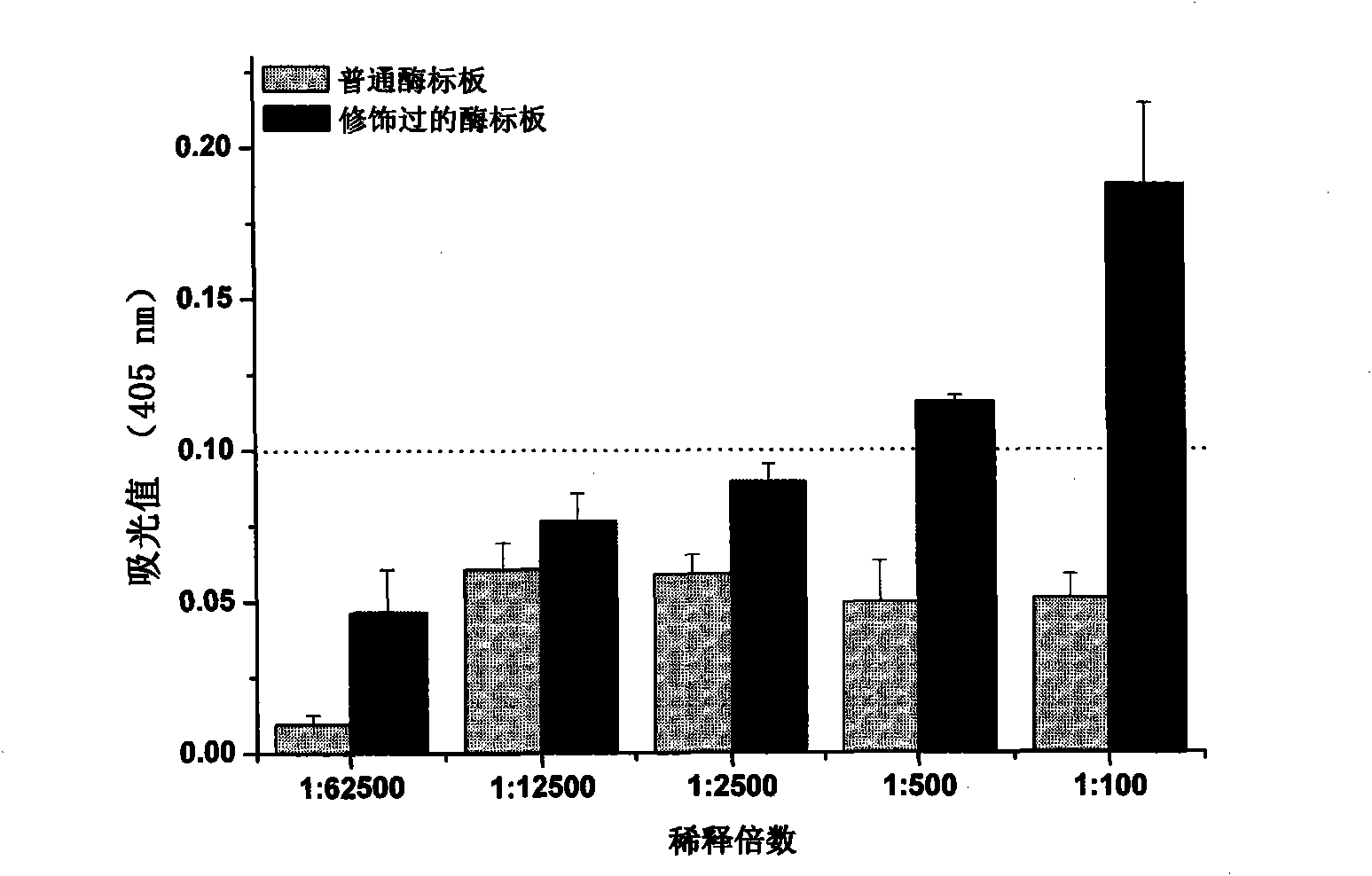
[0034](3) The human plasma was serially diluted with carbonate buffer (pH=9.6). 100 μL per well was coated on ordinary microplates and modified microplates, and incubated in an oven at 37°C for 2 hours. Discard the coating solution in the plate, wash it with plate washing solution for 3 times, then pat dry the residual liquid in the porous plate on absorbent paper, add 300 μL per well of blocking solution containing 1.5% bovine seru...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com