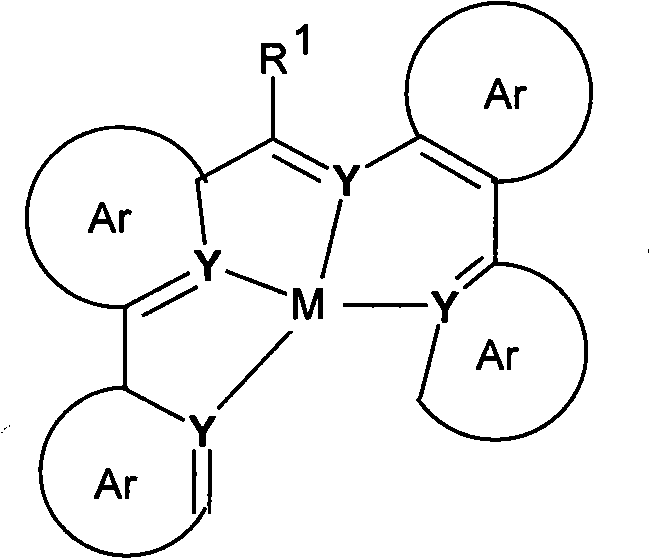
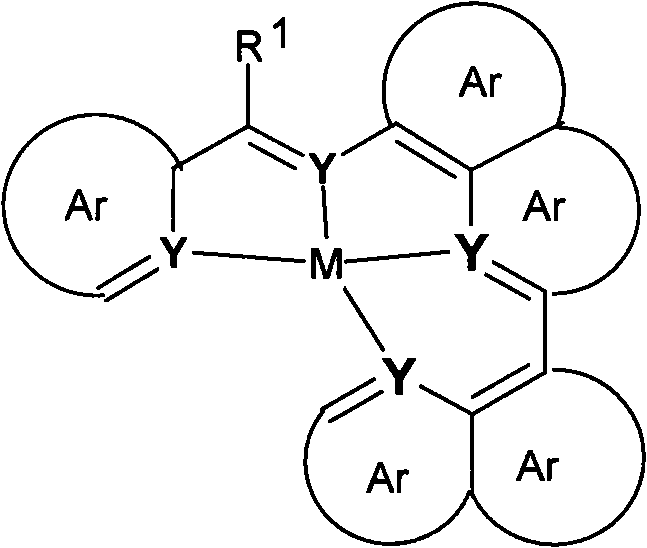
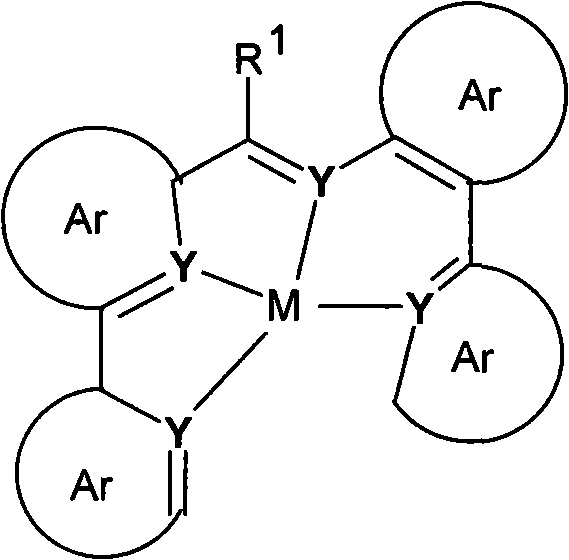
Materials for organic electroluminescent devices
A compound and general formula technology, applied in the field of transition metal complexes, can solve the problems of impossibility, reduced transition efficiency, difficulty in pure primary color, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Embodiment 1: the synthetic steps of metal complex (1)
[0114]
[0115] Step 1: Ligand Synthesis
[0116] The ligand synthesis was carried out using azeotropic distillation to remove the water formed. First, 300 ml of dry toluene was heated to boiling in the distillation apparatus of a 500 ml three-necked flask with stirrer, internal thermometer and dropping funnel. A solution of 20 g (120 mmol) of 2-diphenylamine in 50 ml of dry toluene and a solution of 21.9 g (120 mmol) of 6-phenylpyridine-2-carboxaldehyde in 50 ml of toluene were then slowly added dropwise and sequentially. To this mixture is added a catalytic amount of p-toluenesulfonic acid. Distillation was carried out until clear condensed toluene appeared. Residual solvent was removed in oil pump vacuum (130 Pa). The ligand was recrystallized from toluene and washed with MeOH to afford 31.5 g (94 mmol) of a crystalline solid. The overall yield was 80%.
[0117] Step 2: Complex Synthesis
[0118] The ...
Embodiment 2
[0119] Embodiment 2: the synthetic steps of metal complex (8)
[0120]
[0121] Step 1: Ligand Synthesis
[0122] The ligand synthesis was carried out using azeotropic distillation to remove the water formed. First, 300 ml of dry toluene was heated to boiling in the distillation apparatus of a 500 ml three-necked flask with stirrer, internal thermometer and dropping funnel. A solution of 20 g (120 mmol) of 2-diphenylamine in 50 ml of dry toluene and a solution of 21.9 g (120 mmol) of 3-pyridin-2-ylbenzaldehyde in 50 ml of toluene were then slowly added dropwise and sequentially. To this mixture was added a catalytic amount of p-toluenesulfonic acid. Distillation was carried out until clear condensed toluene appeared. Residual solvent was removed in oil pump vacuum (130 Pa). The ligand was recrystallized from toluene and washed with MeOH to afford 30.1 g (90 mmol) of a crystalline solid. The overall yield was 76%.
[0123] Step 2: Complex Synthesis
[0124] The 1300ml...
Embodiment 3
[0125] Embodiment 3: the synthetic steps of metal complex (30)
[0126]
[0127] Step 1: 8-nitro-2-naphthylquinoline
[0128] Suspend 18.9g (110.0mmol) 1-naphthylboronic acid, 22.9g (110.0mmol) 2-chloro-8-nitroquinoline and 44.6g (210.0mmol) tripotassium phosphate in 500ml toluene, 500ml dioxane and 500ml of water. 913 mg (3.0 mmol) of tri-o-tolylphosphine were added to the suspension, followed by 112 mg (0.5 mmol) of palladium(II) acetate, and the reaction mixture was heated to reflux for 16 h. After cooling, the organic phase is separated off, filtered through silica gel, washed three times with 200 ml of water and evaporated to dryness. Yield 26.4 g (87 mmol), corresponding to 80% of theory.
[0129] Step 2: 8-Amino-2-naphthylquinoline
[0130] 12.6 g (42 mmol) of 8-nitro-2-naphthylquinoline and 1.99 g of Pd / C (10%) were suspended in 200 ml of methanol. 8.4 g (222 mmol) of sodium borohydride were added portionwise with stirring at 0°C. After stirring for 2 h, the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com