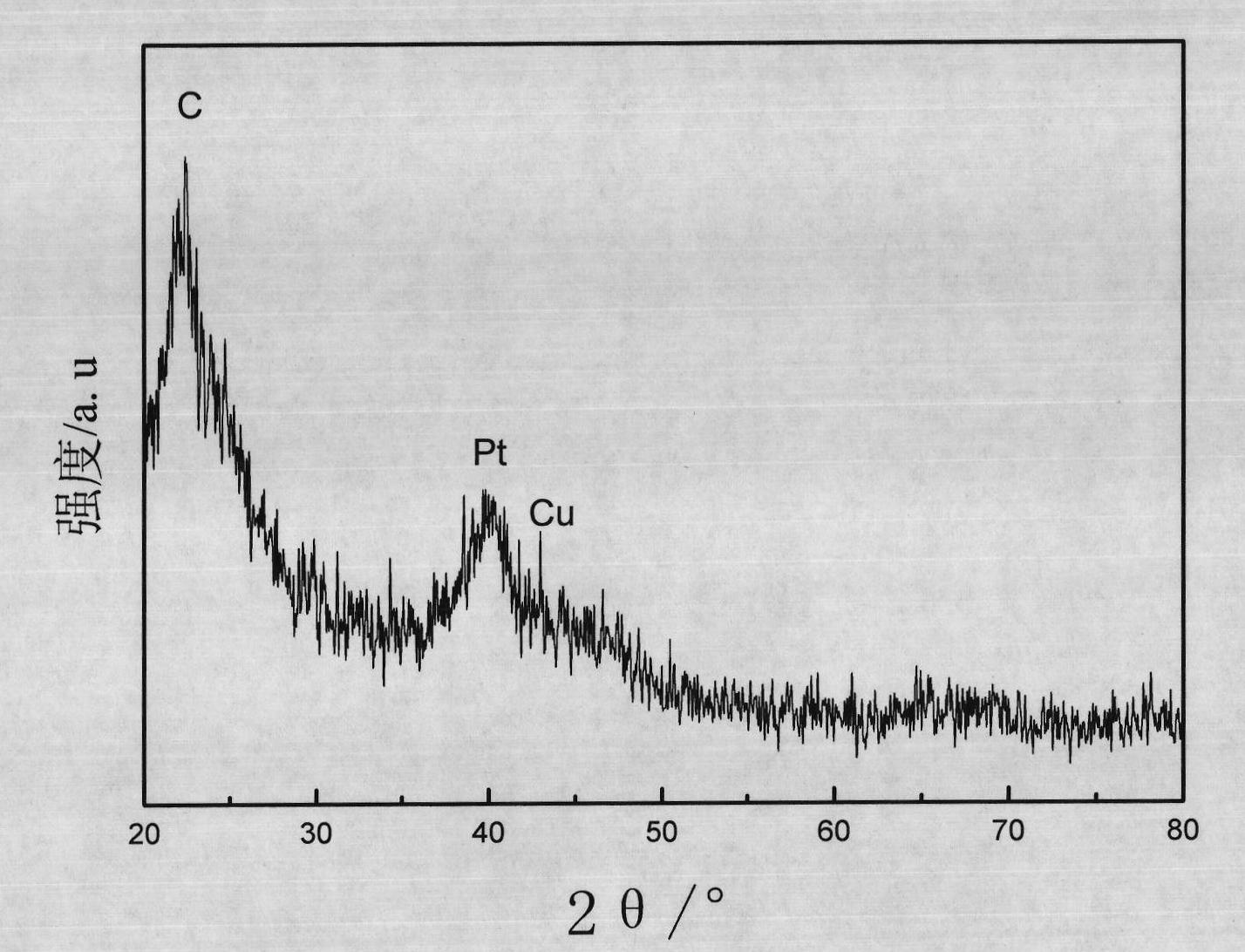
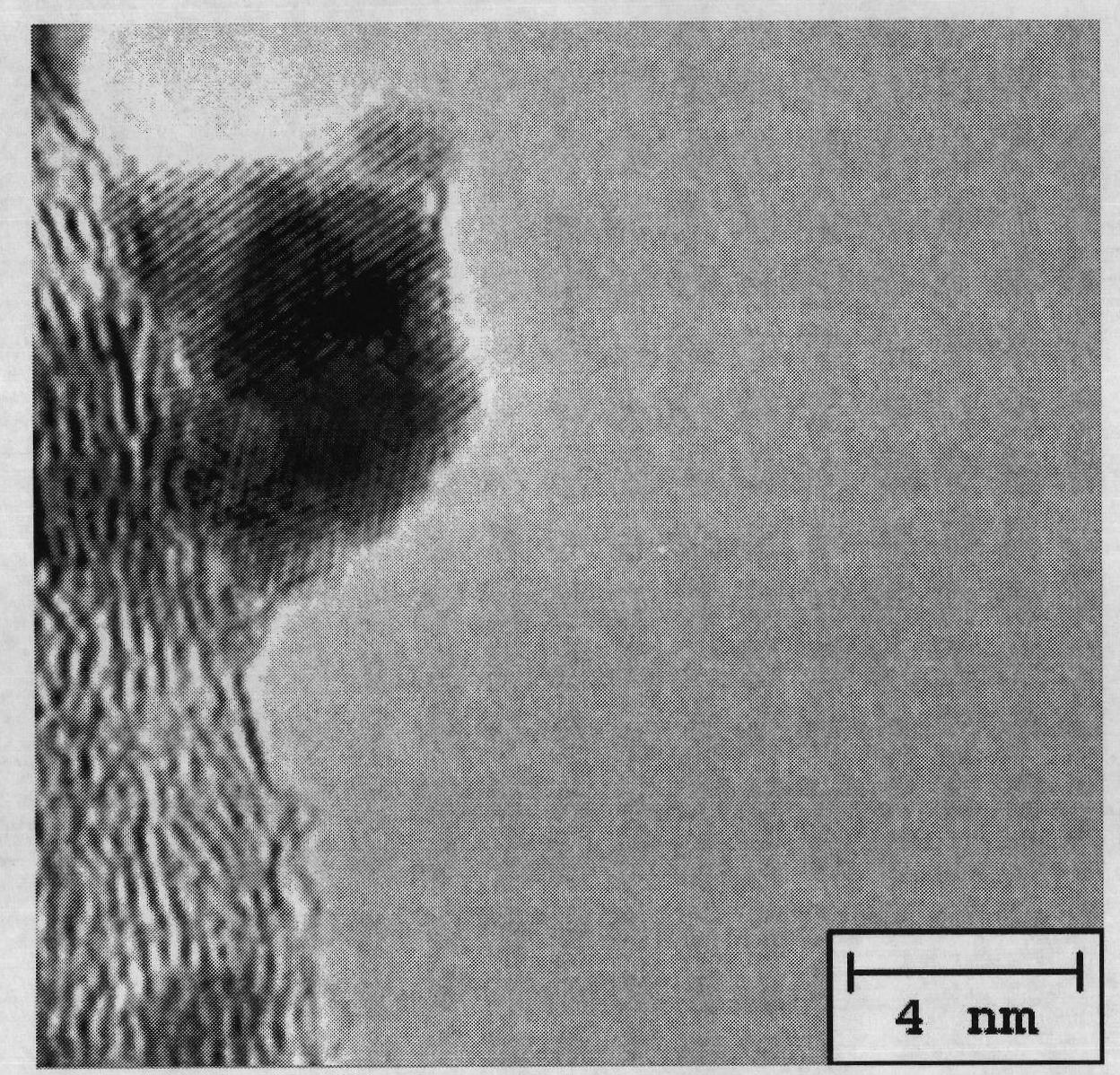
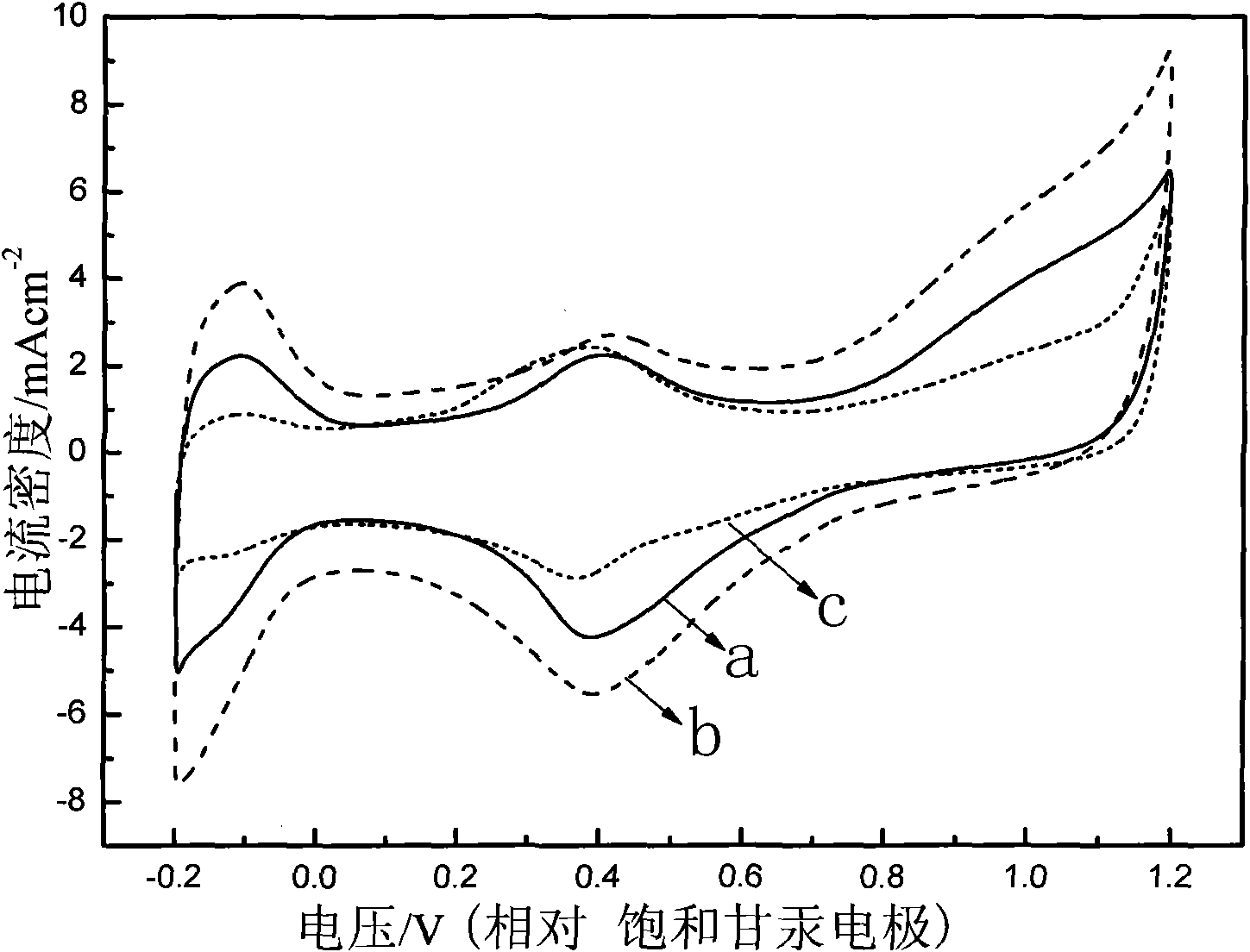
Carbon-carrying shell type copper-platinum catalyst for fuel cell and preparation method thereof
A platinum catalyst, fuel cell technology, used in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. problems, to achieve the effect of enhancing bonding, promoting development, improving catalytic efficiency and utilization of precious metals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029](1) Weigh 39.32mg CuSO 4 ·5H 2 O and 50 mg of citric acid were dissolved in the ethylene glycol solution, after the complete dissolution, the pH value of the system was adjusted to 10 with the ethylene glycol solution of potassium hydroxide, and XC-72 carbon black treated with 80 mg of concentrated nitric acid was added, and stirred thoroughly. ,well mixed;
[0030] (2) The temperature was raised to 160° C. with stirring, and the temperature was kept constant for 6 hours. During the reaction, the ethylene glycol solution of potassium hydroxide was continuously supplemented to keep the pH value of the system constant. After the reaction was completed, the reacted liquid was centrifuged, the precipitate was washed, and dried to constant weight at 60° C. to obtain carbon-supported Cu particles.
[0031] (3) Weigh 90 mg of the product of step B and fully disperse it in the ethylene glycol solution, add 26.54 mg H 2 PtCl 6 ·6H 2 The pH value of the system was adjusted to...
Embodiment 2
[0034] (1) step A with embodiment 1;
[0035] (2) The temperature was raised to 170° C. with stirring, and the temperature was kept constant for 6 hours. During the reaction, the ethylene glycol solution of potassium hydroxide was continuously supplemented to keep the pH value of the system constant. After the reaction was completed, the reacted liquid was centrifuged, the precipitate was washed, and dried to constant weight at 60° C. to obtain carbon-supported Cu particles.
[0036] (3) step C with embodiment 1;
[0037] (4) Same as step D of Example 1, wherein the Cu loading is 10% and the Pt loading is 10%.
Embodiment 3
[0039] (1) step A with embodiment 1;
[0040] (2) The temperature was raised to 180° C. with stirring, and the temperature was kept constant for 6 hours. During the reaction, the ethylene glycol solution of potassium hydroxide was continuously supplemented to keep the pH value of the system constant. After the reaction was completed, the reacted liquid was centrifuged, the precipitate was washed, and dried to constant weight at 60° C. to obtain carbon-supported Cu particles.
[0041] (3) step C with embodiment 1;
[0042] (4) Same as step D of Example 1, wherein the Cu loading is 10% and the Pt loading is 10%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com