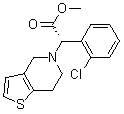
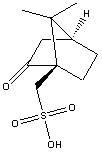
Recycling method of levorotation camphorsulfonic acid serving as clopidogrel resolving agent
A clopidogrel resolving agent and a technology of camphorsulfonic acid are applied in the recovery field of the clopidogrel resolving agent levocamphorsulfonic acid, and can solve the problems of difficult separation of levocamphorsulfonic acid, poor optical purity, complicated process and the like, and achieve The effect of low production cost, short time and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Dissolve 30 g of clopidogrel camphorsulfonate in 120 ml of ethyl acetate and 60 ml of water mixture, adjust the pH value to 8 with saturated sodium carbonate solution, separate the organic layer, and extract the aqueous layer twice with 30 ml of ethyl acetate , discard the ethyl acetate layer, acidify the aqueous layer with concentrated sulfuric acid, adjust the pH value to 0.5, then concentrate and evaporate to dryness to obtain 24 g of solid, add 75 ml of methyl ethyl ketone to the solid and heat to 60 ° C to dissolve the solid, filter, and analyze the filtrate at room temperature crystallized for 12 hours, filtered with suction, and dried the filter cake to obtain 16g of L-camphorsulfonic acid, melting point: 192-193°C, optical rotation: -22.3° (589nm, c=20, H 2 (25°C), yield: 92.0%.
Embodiment 2
[0025] Example 2 Dissolve 30 g of clopidogrel camphorsulfonate in 90 ml of ethyl acetate and 30 ml of water mixture, adjust the pH value to 9 with saturated sodium bicarbonate solution, separate the organic layer, and extract the aqueous layer with 40 ml of ethyl acetate for two Next, discard the ethyl acetate layer, acidify the aqueous layer with concentrated hydrochloric acid until the pH value reaches 1.5, then concentrate and evaporate to dryness to obtain 20 g of a solid, add 160 ml of acetone to the solid and heat to 50 ° C to dissolve the solid, filter, and crystallize the filtrate at room temperature for 10 hour, suction filtration, after the filter cake was dried, 15.5g of L-camphorsulfonic acid was obtained, melting point: 192-194°C, optical rotation: -22.5° (589nm, c=20, H 2 (25°C), yield: 89.1%.
Embodiment 3
[0026] Example 3 Dissolve 30 g of clopidogrel camphorsulfonate in 45 ml of ethyl acetate and 15 ml of water mixture, adjust the pH value to 9 with saturated potassium bicarbonate solution, separate the organic layer, and extract the aqueous layer with 40 ml of ethyl acetate for two The second time, discard the ethyl acetate layer, acidify the aqueous layer with concentrated phosphoric acid until the pH value reaches 3, then concentrate and evaporate to dryness to obtain 25 g of solid, add 50 ml of ethyl acetate to the solid and heat to 50 ° C to dissolve the solid, filter, and analyze the filtrate at room temperature crystallized for 5 hours, filtered with suction, and dried the filter cake to obtain 15.8 g of L-camphorsulfonic acid, melting point: 193-195°C, optical rotation: -21.8° (589nm, c=20, H 2 (25°C), yield: 90.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com