Azobenzene polypeptide block copolymer and preparation method and application thereof
A block copolymer, azobenzene technology, applied in the field of compound synthesis and preparation, can solve difficult azobenzene functional polypeptide block copolymer, difficult to synthesize, azobenzene functional polypeptide block copolymerization Less material and other problems, to achieve the effect of a wide range of application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
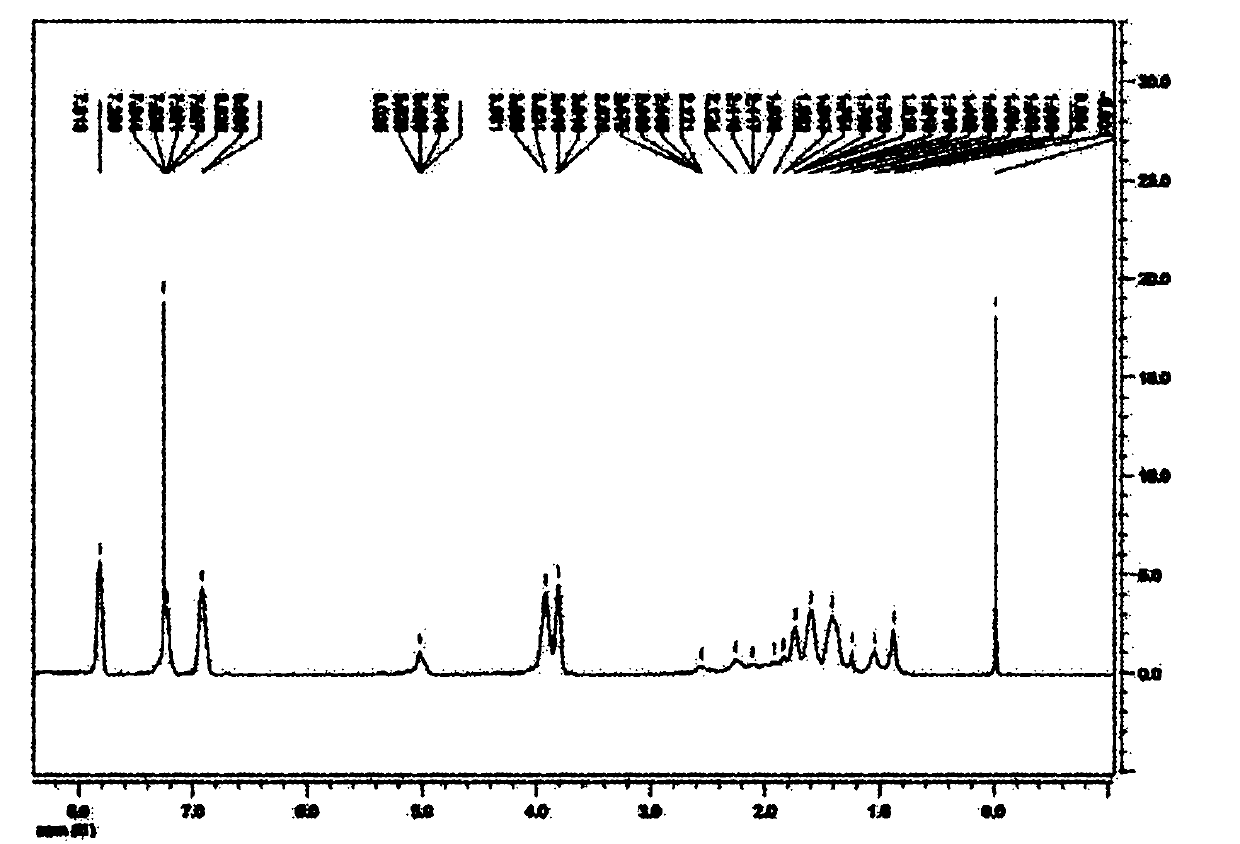
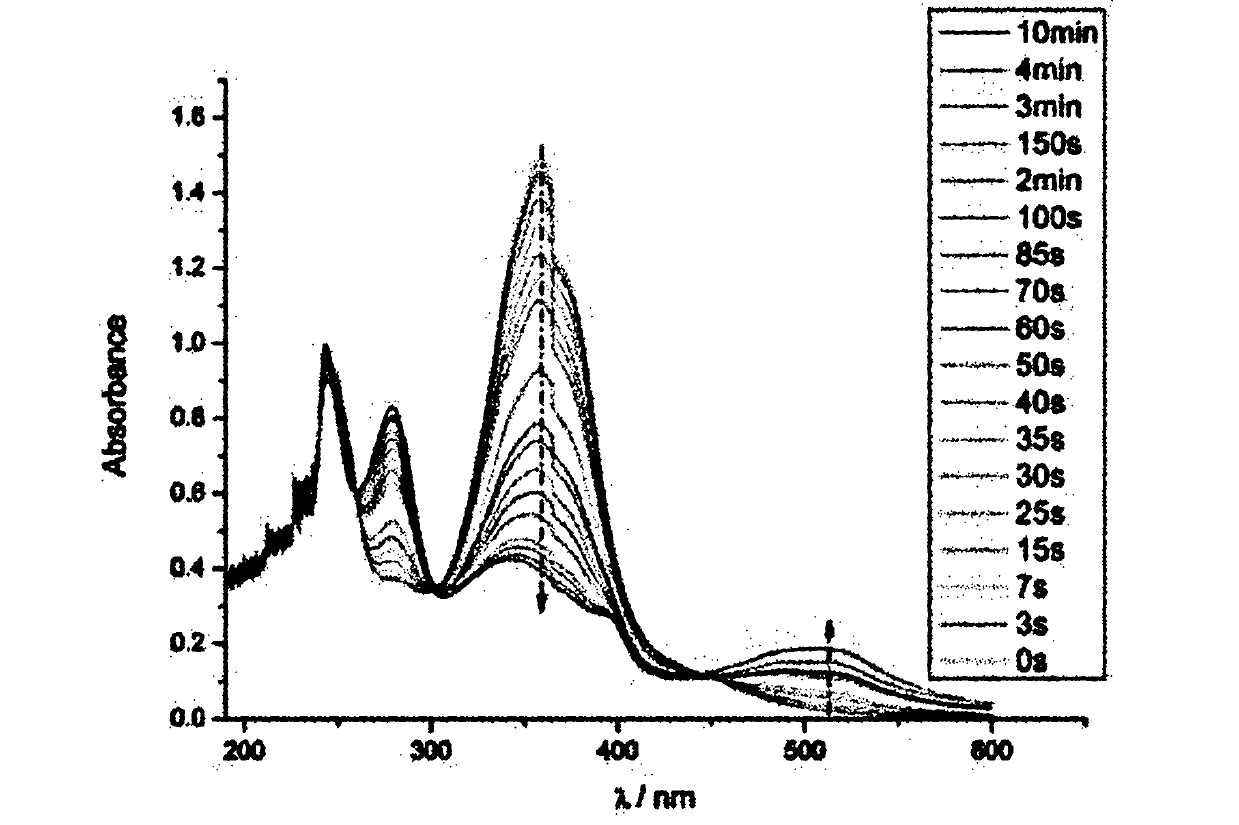
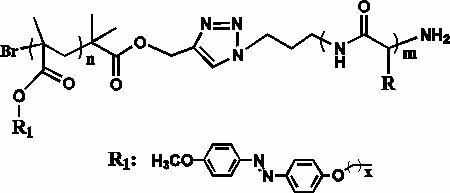
[0033] Example Preparation of a kind of azobenzene polypeptide block copolymer
[0034] (1) homopolymerization process
[0035] ① Synthesis of polymers containing azophenyl groups
[0036] In a 10ml polymerization tube, the monomer 6-(4'-methoxy-4-oxoazobenzene)-hexyl methacrylate (0.340g, 0.01mmol) solution was dissolved in 4.5mL chlorobenzene, and the After nitrogen deoxygenation for 30 minutes, the initiator α-propynyl bromoisobutyrate (5.6mg, 0.03mmol) was added and reacted with cuprous bromide, N,N,N,N,N-pentamethyldiethyl The catalyst system composed of triamine (PMDETA) was sealed at 85°C for 10 hours, then precipitated with ether three times, filtered by suction, and dried in vacuum to obtain an orange solid polymer containing azophenyl groups with a yield of 90%. ;
[0037] ② Polypeptide synthesis process
[0038] Under the protection of nitrogen, γ-benzyl-L-glutamate (1.0g, 5.4mmol), triphosgene (0.53g, 1.8mmol) and ethyl acetate 30ml were added to a 100ml thre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com