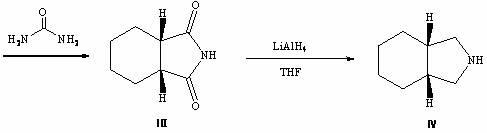
Preparation method of cis-hexahydroisoindoline
A kind of hexahydroisoindole, cis technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
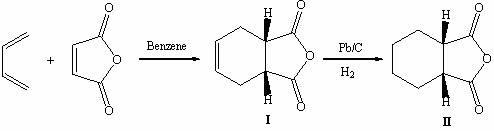
[0019] Step 1, the synthesis of cis-1,2,3,6-tetrahydrophthalic anhydride
[0020] Add maleic anhydride (98.0 g, 1.000 mol) into a reactor filled with dry benzene (250 mL), and feed 1,3-butadiene at a constant speed (0.6-0.8 L / min) under heating and stirring, and the reaction temperature After reaching 50 °C, heat for another 3 min, then stop heating, continue to feed 1,3-butadiene, react for 3.5 h, GC detects that the raw material maleic anhydride disappears, stop the reaction, stand at 0~5 °C for 12 h to crystallize , suction filtration, washing with petroleum ether, drying at ≤60 ℃ to constant weight, yield: 96.7%;
[0021] Step 2, the synthesis of cis-cyclohexanedicarboxylic anhydride
[0022] Dissolve cis-1,2,3,6-tetrahydrophthalic anhydride (178.3 g, 1.173 mol) in methanol (2000 mL), add 10% Pd / C (35.6 g), first vacuum, then use Replaced with hydrogen 3 times, introduced hydrogen, hydrogenated at normal pressure for 22 hours, filtered the reaction solution with suction,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com