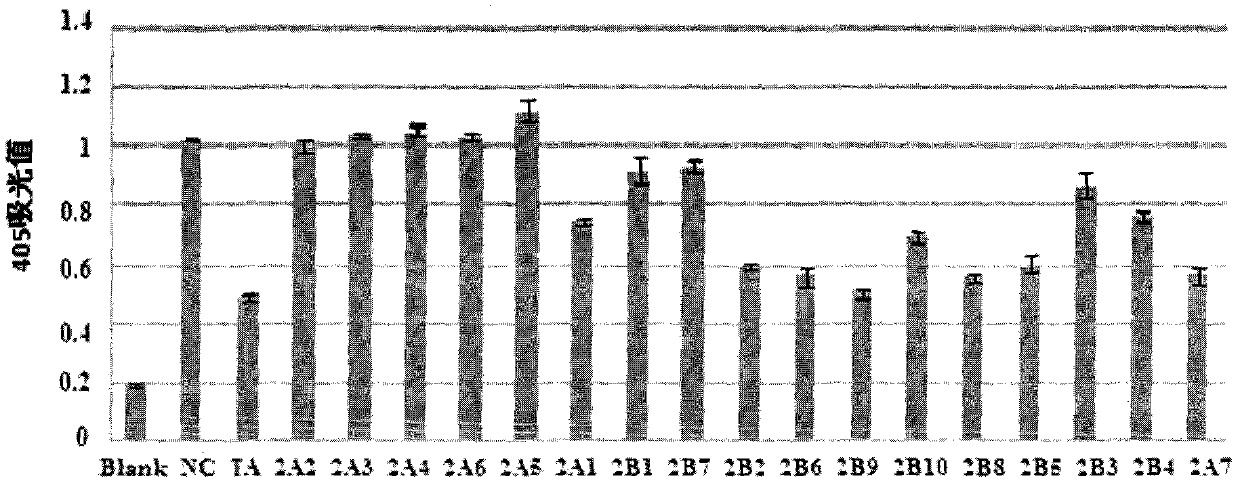
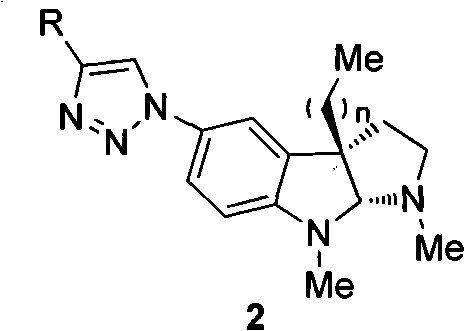
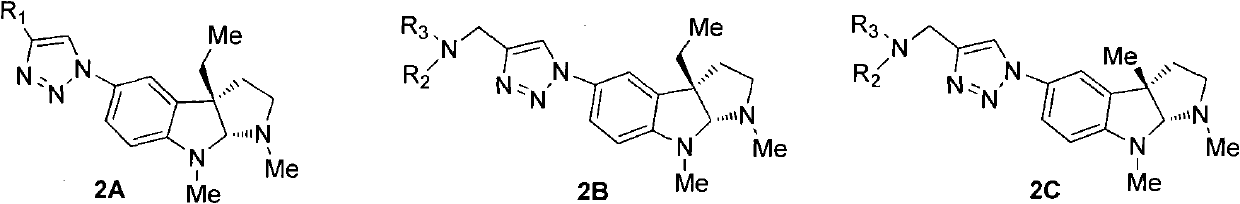
Preparation method of physostigmine derivatives with resistance to activity of senile dementia disease
A technology for inhibiting activity and substances, applied in the field of synthetic derivatives of physostigmine, which can solve the problems of short action time, narrow therapeutic window, and low bioavailability, and achieve the effect of short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of Implementation Example 1 Compound 6
[0035]
[0036] After trifluoroacetic acid (1.6mL, 21.61mmol) and compound 5 (2.36g, 8.28mmol) were stirred at room temperature for 10 minutes, compound 3 (12.0g, 51.72mmol), 34mL of dichloromethane and 6mL of water were added successively, and the reaction The liquid was cooled to -78°C, and acrolein 4 (35 mL, 0.52 mol) was slowly added. After the completion of the reaction was monitored by TLC, the reaction solution was raised to 0° C., and the reaction was quenched by adding a phosphate buffer solution with pH=7. Extracted with diethyl ether (3×50mL), combined the organic phases, dried with anhydrous sodium sulfate, filtered and sucked dry to obtain an orange-yellow solid, which was subjected to silica gel column chromatography (20% EA / PE) to obtain compound 6 (13.0 g, yield rate 87%). 1 H NMR (400MHz, CDCl 3 )δ1.97(m, 2H), 2.13-2.16(m, 2H), 2.2-2.8(m, 1H), 2.42(m, 1H), 2.89(s, 1H), 2.98(s, 3H), 3.71 (s, 3H), ...
Embodiment 2
[0037] Implementation Example 2 Synthesis of Compound 7
[0038]
[0039] Compound 6 (12.0 g, 41.67 mmol) was dissolved in 100 mL of dry tetrahydrofuran, and lithium aluminum tetrahydride (9.5 g, 0.25 mol) was added in batches under an ice bath, and the reaction solution was heated to reflux. After the completion of the reaction was monitored by TLC, a saturated sodium sulfate aqueous solution was added dropwise to the reaction liquid under an ice bath until the white solid no longer increased, filtered, and the filter residue was washed repeatedly with ethyl acetate, and the organic phases were combined and drained. Recrystallized 3 times with ether, the mother liquor was concentrated and dried by silica gel column chromatography (5-10% MeOH / CH 2 Cl 2 ) to obtain compound 7 (9.8 g, yield 95.%). M.p.71-72℃; 1 H NMR (400MHz, CDCl 3 )δ1.31(m, 1H), 1.47(m, 1H), 1.75(dd, J=12, 4.4Hz, 1H), 1.85(dd, J=12, 4.4Hz, 1H), 1.95(m, 1H ), 2.02(m, 1H), 2.51(s, 3H), 2.53(m, 1H), 2.69(...
Embodiment 3
[0040] Implementation Example 3 Synthesis of Compound 1a
[0041]
[0042] Compound 7 (6.0 g, 24.39 mmol) was dissolved in 100 mL of dry toluene, 60.0 g of Raney nickel was added, and the reaction solution was refluxed for water separation, then heated to reflux for 4 hours. After TLC monitors that the reaction is complete, the Raney nickel is filtered off, the filter residue is washed repeatedly with ethyl acetate, the organic phases are combined, drained, and the crude product is separated and purified by silica gel column chromatography (1-5% MeOH / CH 2 Cl 2 ), to obtain compound 1a (2.68 g, yield 51%). 1 H NMR (400MHz, CDCl 3 )δ0.75(t, J=7.2Hz, 3H), 1.70(sext, J=7.2Hz, 1H), 1.82(sext, J=7.2Hz, 1H), 1.93(m, 1H), 2.01(m, 1H), 2.52(s, 3H), 2.55(m, 1H), 2.68(m, 1H), 2.94(s, 3H), 4.16(s, 1H), 6.41(d, J=7.6Hz, 1H), 6.67(t, J=7.6Hz, 1H), 6.95(d, J=7.6Hz, 1H), 7.08(t, J=7.6Hz, 1H)ppm; 13 C NMR (100MHz, CDCl 3 )δ10.0, 32.5, 36.4, 37.8, 39.3, 52.6, 57.5, 93.8, 106.5, 117.5, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com