Method for preparing cobaltosic oxide nanorod by using microemulsion
A technology of cobalt trioxide and microemulsion method, which is applied in the field of Co3O4 porous nanorods and Co3O4 nanorods, which can solve the problems of long process, unsuitable for large-scale industrial production, and complicated experimental equipment, and achieve uniform particle size distribution, cheap experimental raw materials, Simple Effects of Experimental Equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
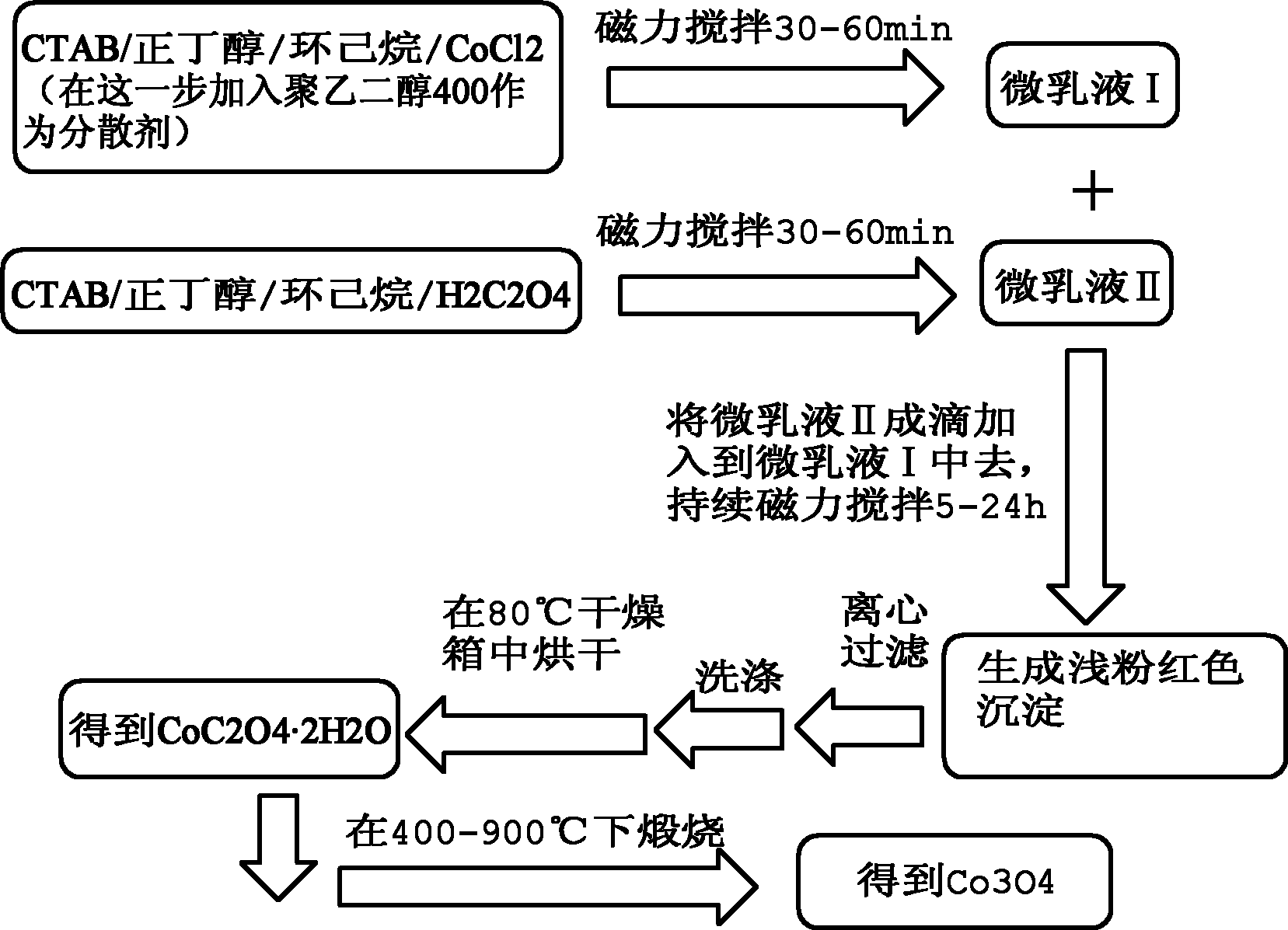
[0046] 1) Weigh 0.8328g of CoCl 2 ·6H 2 O (analytically pure, content ≥ 99.0%), add 70mL deionized water to prepare 0.05M CoCl 2 aqueous solution;
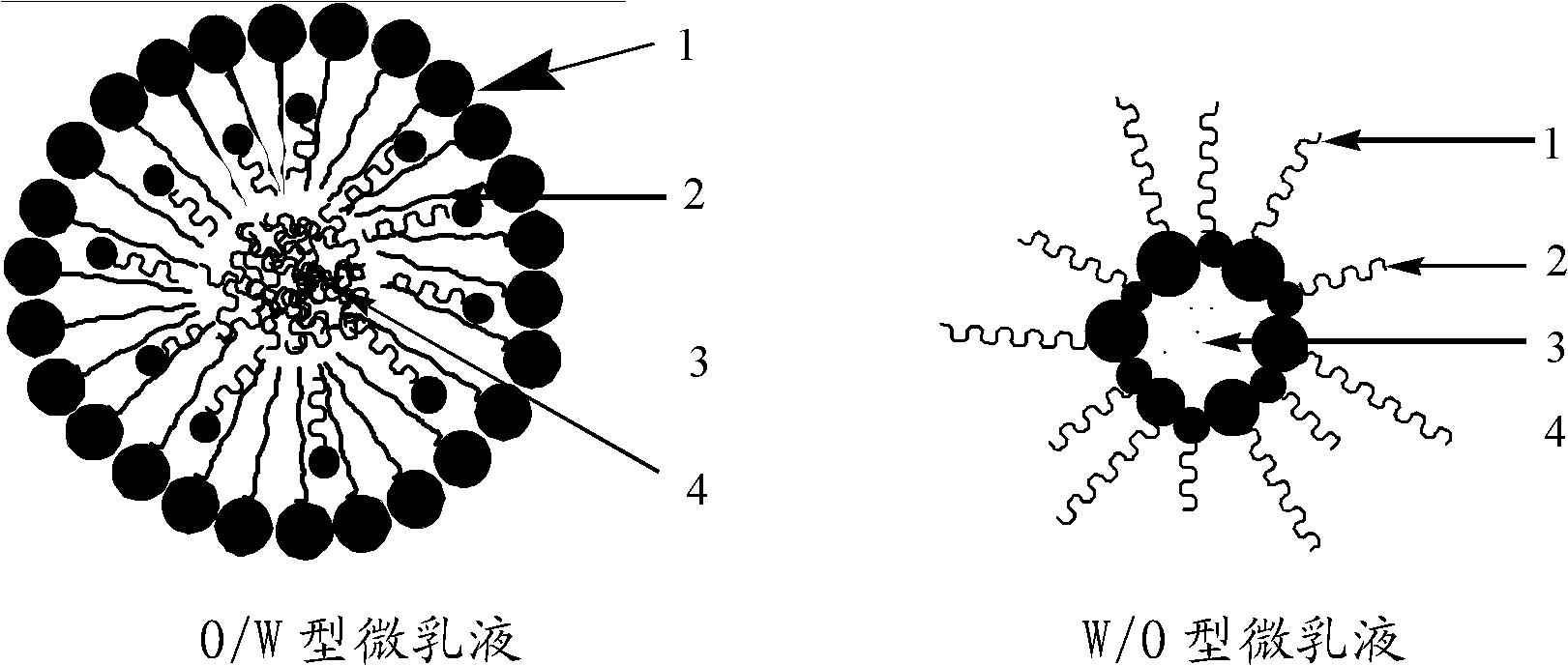
[0047] 2) Add 5g CTAB (analytical pure, content ≥99.0%), 2.5g n-butanol (analytical pure, content ≥99.0%), and 5g cyclohexane (analytical pure, content ≥99.5%) to the prepared CoCl 2 The solution was magnetically stirred at room temperature for 30-60 minutes, and the solution was observed to change from turbid to transparent, and microemulsion I was prepared.
[0048]3) add wherein account for the whole microemulsion system (this system is the summation of microemulsion I and microemulsion II, excluding polyethylene glycol) gross mass 40% polyethylene glycol (chemically pure, content ≥ 99.9%), The dropping rate is 2mL / min, stir evenly;
[0049] 4) Weigh 0.6304g of H 2 C 2 o 4 2H 2 O (analytically pure, content ≥ 99.5%), add 50mL deionized water to prepare 0.1M H 2 C 2 o 4 aqueous solution;
[0050] 5) 5gCTAB (analytica...
Embodiment 2
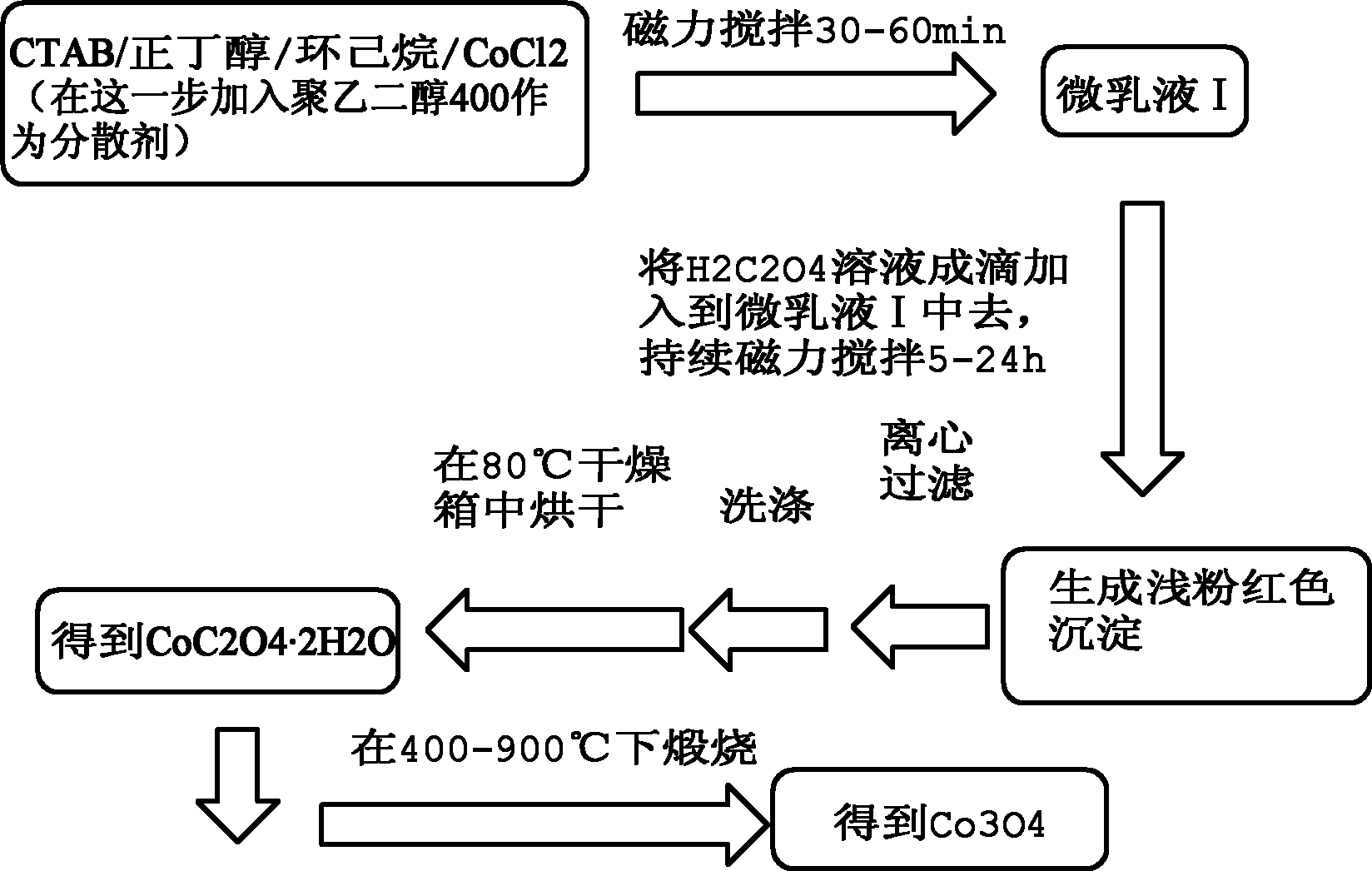
[0056] 1) Weigh 0.8328g of CoCl 2 ·6H 2 O (analytically pure, content ≥ 99.0%), add 70mL deionized water to prepare 0.05M CoCl 2 aqueous solution;
[0057] 2) Add 5g CTAB (analytical pure, content ≥99.0%), 2.5g n-butanol (analytical pure, content ≥99.0%), and 5g cyclohexane (analytical pure, content ≥99.5%) to the prepared CoCl 2 The solution was magnetically stirred at room temperature for 30-60 minutes, and the solution was observed to change from turbid to transparent, and microemulsion I was prepared.
[0058] 3) adding thereto accounted for the whole microemulsion system (this system is the summation of microemulsion I and microemulsion II, excluding polyethylene glycol) gross mass 20% polyethylene glycol (chemically pure, content ≥ 99.9%), The dropping rate is 2mL / min, stir evenly;
[0059] 4) Weigh 0.6304g of H 2 C 2 o 4 2H 2 O (analytically pure, content ≥ 99.5%), add 50mL deionized water to prepare 0.1M H 2 C 2 o 4 aqueous solution;
[0060] 5) 5gCTAB (ana...
Embodiment 3
[0066] 1) Weigh 0.8328g of CoCl 2 ·6H 2 O (analytically pure, content ≥ 99.0%), add 70mL deionized water to prepare 0.05M CoCl 2 aqueous solution;
[0067] 2) Add 5g CTAB (analytical pure, content ≥99.0%), 2.5g n-butanol (analytical pure, content ≥99.0%), and 5g cyclohexane (analytical pure, content ≥99.5%) to the prepared CoCl 2 The solution was magnetically stirred at room temperature for 30-60 minutes, and the solution was observed to change from turbid to transparent, and microemulsion I was prepared.
[0068] 3) add wherein account for the whole microemulsion system (this system is the summation of microemulsion I and microemulsion II, excluding polyethylene glycol) gross mass 30% polyethylene glycol (chemically pure, content ≥ 99.9%), The dropping rate is 2mL / min, stir evenly;
[0069] 4) Weigh 0.6304g of H 2 C 2 o 4 2H 2 O (analytically pure, content ≥ 99.5%), add 50mL deionized water to prepare 0.1M H 2 C 2 o 4 aqueous solution;
[0070] 5) 5gCTAB (analytic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com