Allylation method of aromatic hydrocarbon
A technology for allylation and aromatic hydrocarbons, applied in the field of organic synthesis, can solve the problems of expensive catalyst, increase reaction cost, and high equipment requirements, and achieve the effects of simple structure, cost saving, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
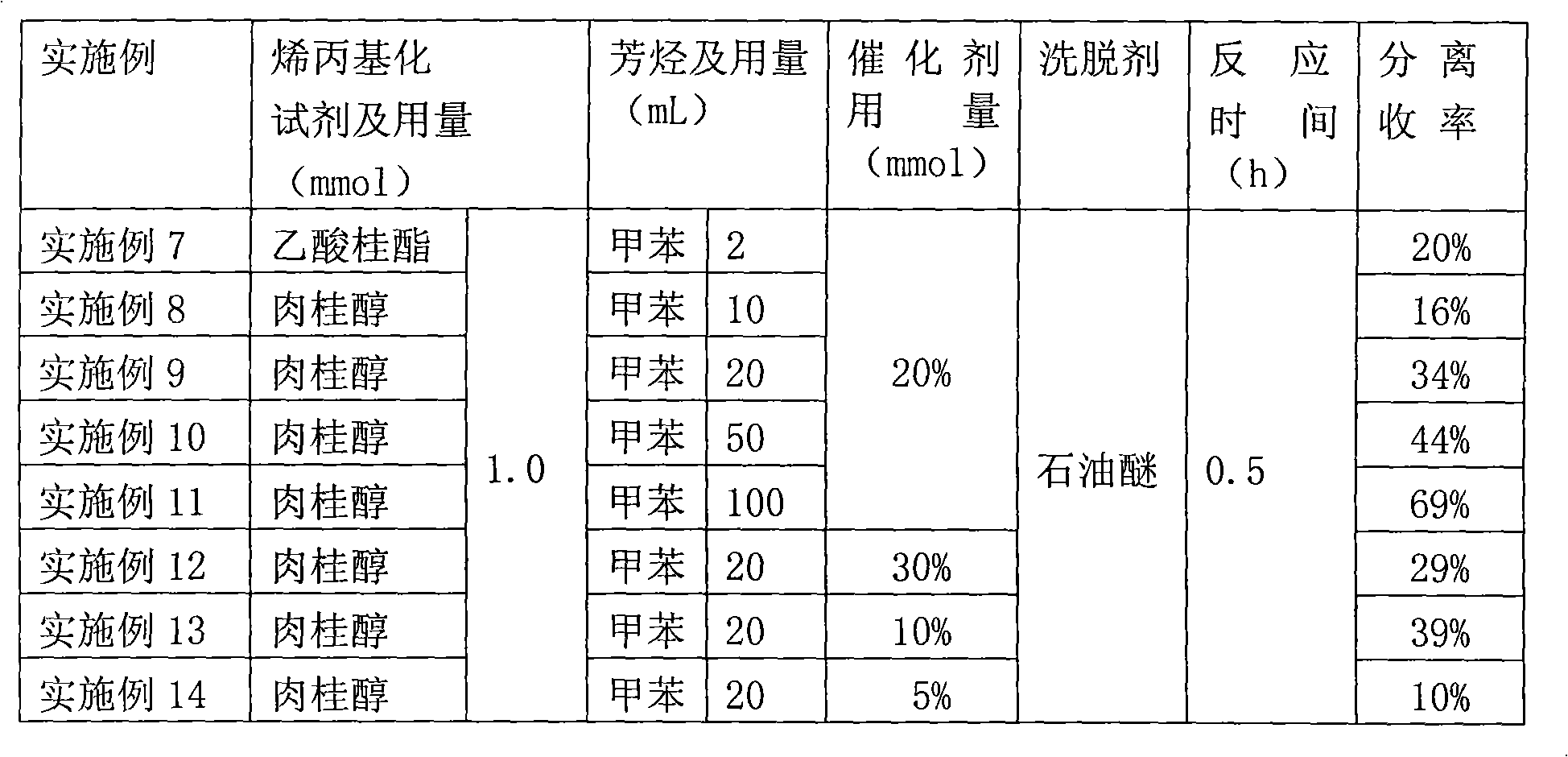
Examples
Embodiment 1
[0025] a FeCl 3 ·6H 2 O catalyzes the method for the synthetic allylation product of aromatic hydrocarbons, and its concrete steps are as follows:
[0026] (1) Toluene is used as a raw material and used as a solvent, and FeCl 3 ·6H 2 O is a catalyst, with cinnamyl acetate as an allylation reagent, according to cinnamyl acetate (mmol): FeCl 3 ·6H 2 The ratio of O (mmol):toluene (ml) is 1:0.2:50, and FeCl is first added to the reactor 3 ·6H 2 O (54.06mg, 0.1mmol) catalyst and cinnamyl acetate (176.21mg, 0.1mmol) were added, and toluene (50mL) was added. After the addition was completed, the temperature was raised to 80°C, and the reaction was carried out under stirring for 0.5 hours.
[0027] (2) After the first (1) step is completed, the allylation product reaction liquid prepared in the (1) step is cooled in the air, and the condensing system of the reactor is rinsed with dichloromethane, and the washing liquid is incorporated into the reactor in the reaction solution. ...
Embodiment 2
[0029] a FeCl 3 ·6H 2 O catalyzes the method for the synthetic allylation product of aromatic hydrocarbons, and its specific steps are with embodiment 1, wherein:
[0030] In step (1), take o-xylene as raw material and do solvent concurrently, use FeCl 3 ·6H 2 O is a catalyst, with cinnamyl acetate as an allylation reagent, according to cinnamyl acetate (mmol): FeCl 3 ·6H 2 The ratio of O (mmol): o-xylene (ml) is 1: 0.2: 50, FeCl is first added to the reactor 3 ·6H 2 O (54.06mg, 0.1mmol) catalyst and cinnamyl acetate (176.21mg, 0.1mmol) were added, and o-xylene (50mL) was added. After the addition was completed, the temperature was raised to 80°C, and the reaction was carried out under stirring for 0.5 hours.
[0031] In step (2), cinnamyl xylene (167.1 mg, yield 75%) was obtained as a colorless transparent liquid.
Embodiment 3
[0033] a FeCl 3 ·6H 2 O catalyzes the method for the synthetic allylation product of aromatic hydrocarbons, and its specific steps are with embodiment 1, wherein:
[0034] In step (1), take m-xylene as raw material and do solvent concurrently, use FeCl 3 ·6H 2 O is a catalyst, with cinnamyl acetate as an allylation reagent, according to cinnamyl acetate (mmol): FeCl 3 ·6H 2 The ratio of O (mmol): m-xylene (ml) is 1: 0.2: 50, FeCl is first added to the reactor 3 ·6H 2 O (54.06mg, 0.1mmol) catalyst and cinnamyl acetate (176.21mg, 0.1mmol) were added, m-xylene (50mL) was added, the temperature was raised to 80°C, and the reaction was carried out under stirring for 0.5 hours.
[0035] In step (2), cinnamyl xylene (140.5 mg, yield 63%) was obtained as a colorless transparent liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com