Medicinal composition containing realgar
A composition and technology of realgar, applied in the field of anti-tumor drugs, can solve the problems of reducing toxicity, achieve low toxicity and improve the effect of anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The preparation and quality control of each component of embodiment 1 medicine
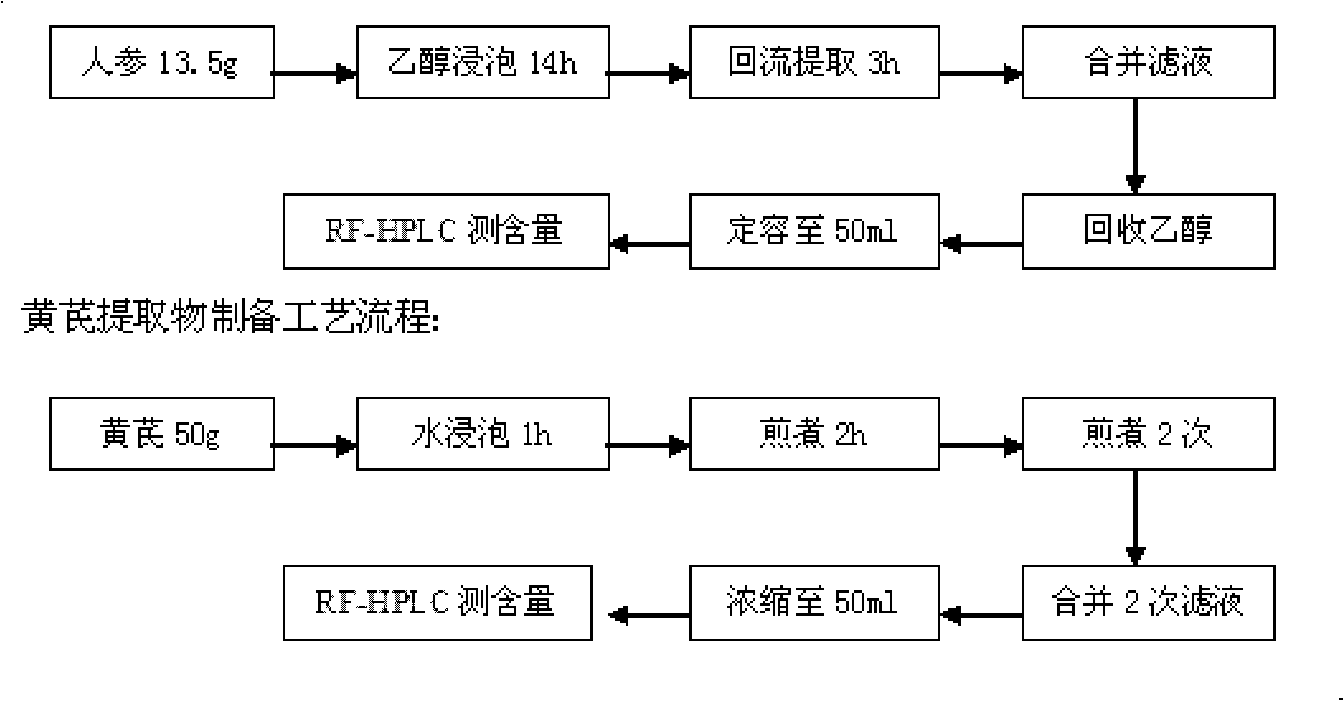
[0017] Preparation of realgar dissolving solution: prepared according to the bacterial treatment method disclosed in Chinese invention patent application number 2006102000675. Use the leaching microorganism Thiobacillus ferrooxidans to process the realgar powder biologically. The realgar is at a room temperature of about 30°C, the particle size is 180-200 mesh, the pulp concentration is 1%, and the initial Fe 2+ The concentration is 1.0g / L, the pH value of the slurry is 2.2, the bacterial inoculum is 20%, the flask is shaken at 130rpm for 30 days, filtered, and the filtrate is the realgar solution. The total arsenic content of the solution was determined to be 1590 μg / ml by inductively coupled plasma atomic emission spectrometry (ICP-AES). Dilute to 32μg / ml before use, and set aside.
[0018] Preparation of matrine solution: take 50 mg of matrine, dissolve in 5 ml of distilled water to ma...
Embodiment 2
[0022] The inhibitory action of embodiment two realgar dissolving solution to nematode Let-60
[0023] Material: C. elegans: Let-60(n1046)IV
[0024] M solution: 15g Na per liter of buffer 2 HPO 4 12H 2 O, 3g KH 2 PO 4 , 5g NaCl, 0.25g MgSO 4 ·7H 2 O.
[0025] Principle: Studies have found that about 30% of human tumors have mutation activation of Ras gene and increased expression of Ras protein (Signal-Transduction[M].2005: 20-100). source let-60 Caenorhabditis elegans mutant, the effects of two Ras farnesyltransferase inhibitors on the Ras pathway were investigated, and the results showed that the two Ras farnesyltransferase inhibitors can downregulate the hyperactivity of the Ras pathway Activated, let-60 mutant nematodes with a polyvulva phenotype become wild-type under the action of drugs (PNAS 1995:92:3333-3337.). Therefore, Caenorhabditis elegans polyvulva phenotype mutants have been widely adopted as model organisms for anti-cancer gene Ras active drug screeni...
Embodiment 3
[0034] The effect of embodiment three realgar dissolving liquid compositions on Let-60
[0035] Experimental procedure: with embodiment two
[0036] LC50 experimental procedure: same as embodiment two
[0037] Composition of realgar dissolving solution: 40% realgar dissolving solution, 0.8% matrine, 0.8% ginseng extract, 0.8% astragalus extract, M solution supplemented to 100%. The experimental results are shown in Table 2:
[0038] The effect of table 2 realgar dissolving solution composition on Let-60
[0039] group
[0040] Note: Compared with the blank group *P<0.05
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com