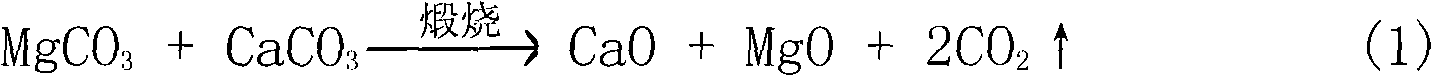Process for removing calcium from magnesite
A magnesite and calcium process technology, applied in the direction of magnesium hydroxide, etc., can solve the problems of incomplete calcium removal, long process, and reduction to 1.5%, and achieve the effect of flexible product solutions, short process flow, and thorough calcium removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Take 500g of magnesite containing 8.71% CaO and place it in a muffle furnace, and calcinate at 940°C for 2h to obtain 240g of light-burned magnesium oxide powder. Put the calcined light-calcined magnesium oxide powder in a 1000mL reaction tank, add 500mL of 30°C water, and carry out the digestion reaction under stirring. After 20 minutes, 90 g of magnesium nitrate was added thereto, and the stirring was continued for 30 minutes to carry out the decalcification reaction, and the reaction temperature during the decalcification process was maintained at 50° C. After the reaction, the slurry was filtered, and the filter cake was washed with 1260 mL of water. The washed filter cake was dried in an oven at 110° C. for 4 hours to obtain 306 g of magnesium hydroxide product, and the CaO content in the magnesium hydroxide product was 0.42%.
Embodiment 2
[0032] Take 500g of magnesite containing 9.32% CaO and place it in a muffle furnace, and calcinate at 990°C for 2h. 240 g of lightly burned magnesium oxide powder was obtained. Put the calcined light-calcined magnesium oxide powder in a 2000mL reaction tank, add 1000mL of 50°C water, and carry out the digestion reaction under stirring. After 15 minutes, 141 g of magnesium chloride hexahydrate was added thereto, and the stirring was continued for 1.5 hours to carry out the decalcification reaction, and the reaction temperature during the decalcification process was maintained at 70°C. After the reaction, the slurry was filtered, and the filter cake was washed with 1820 mL of water. The washed filter cake was dried in an oven at 120° C. for 3 hours to obtain 306 g of magnesium hydroxide product, and the CaO content in the magnesium hydroxide product was 1.13%.
Embodiment 3
[0034] Take 500g of magnesite containing 7.13% CaO, place it in a muffle furnace, and calcinate it at 1030°C for 2h. 241 g of lightly burned magnesium oxide powder was obtained. Put the lightly calcined magnesium oxide powder obtained by calcining into a 2000mL reaction tank, add 1400mL of 20°C water, and carry out the digestion reaction under stirring. After 1.5 hours, 118 g of magnesium bromide was added thereto, and the stirring was continued for 2 hours to carry out the decalcification reaction, and the reaction temperature during the decalcification process was maintained at 30° C. After the reaction, the slurry was filtered, and the filter cake was washed with 3200 mL of water. The washed filter cake was dried in an oven at 130° C. for 2 hours to obtain 307 g of magnesium hydroxide product, and the CaO content in the magnesium hydroxide product was 0.29%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com