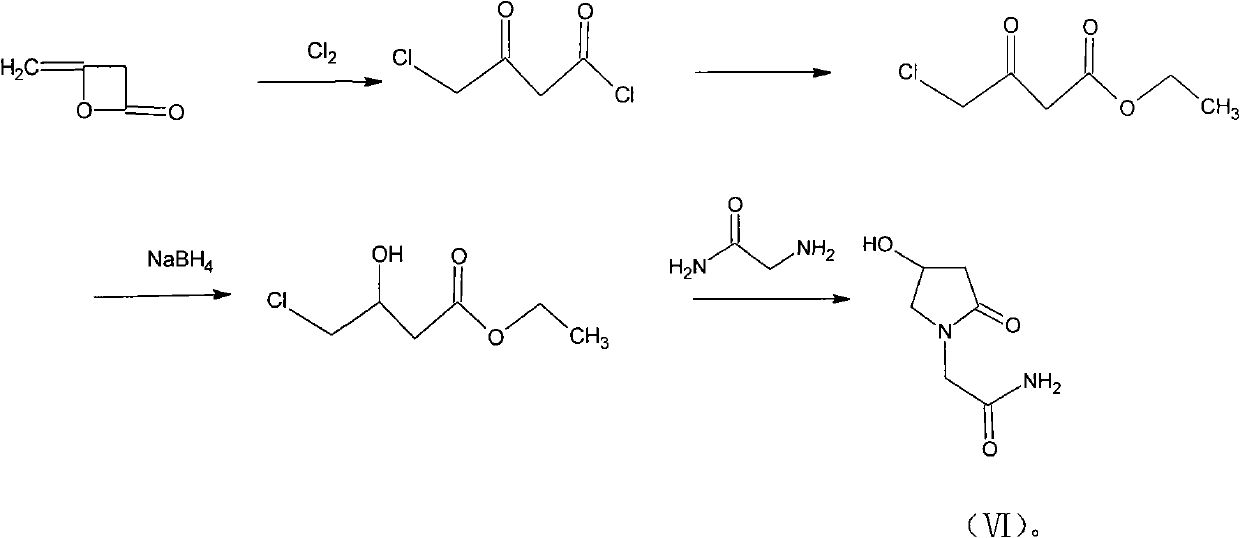
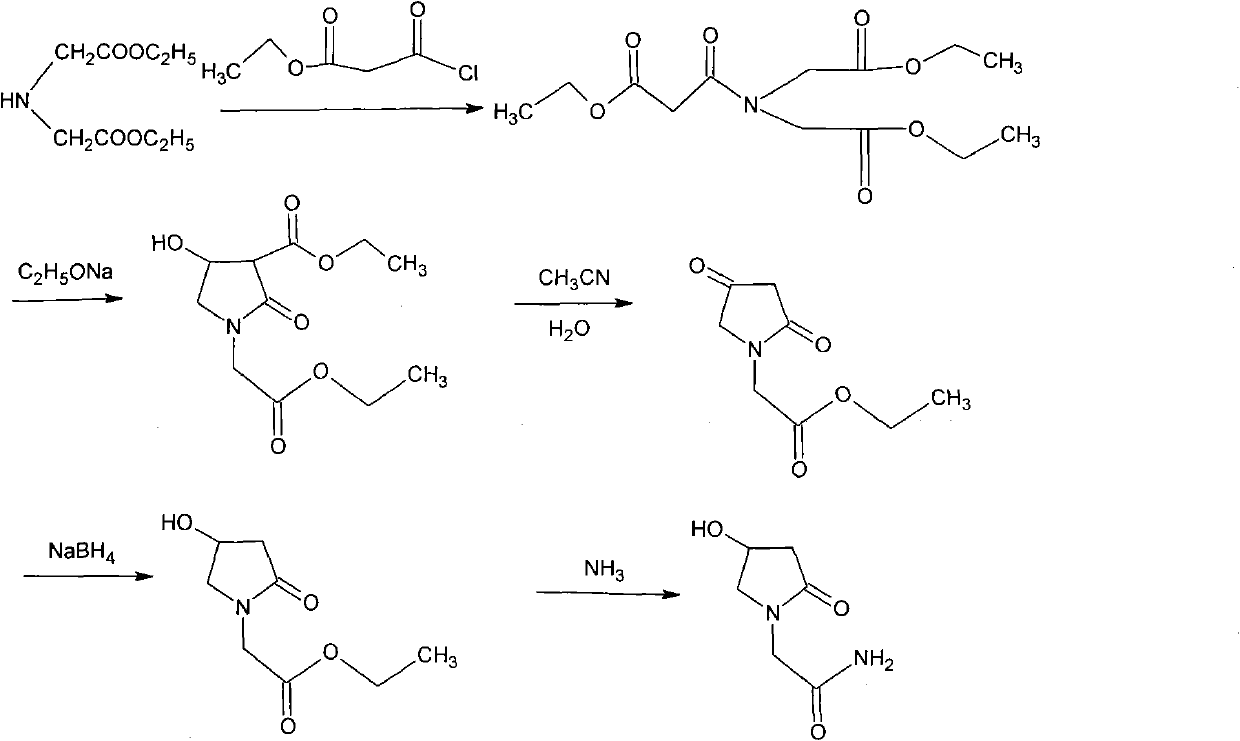
Preparation method of Oxiracetam
A technique called ammonolysis reaction, applied in the direction of organic chemistry, etc., can solve the problems of affecting the quality of oxiracetam, high price of sodium azide, and explosiveness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
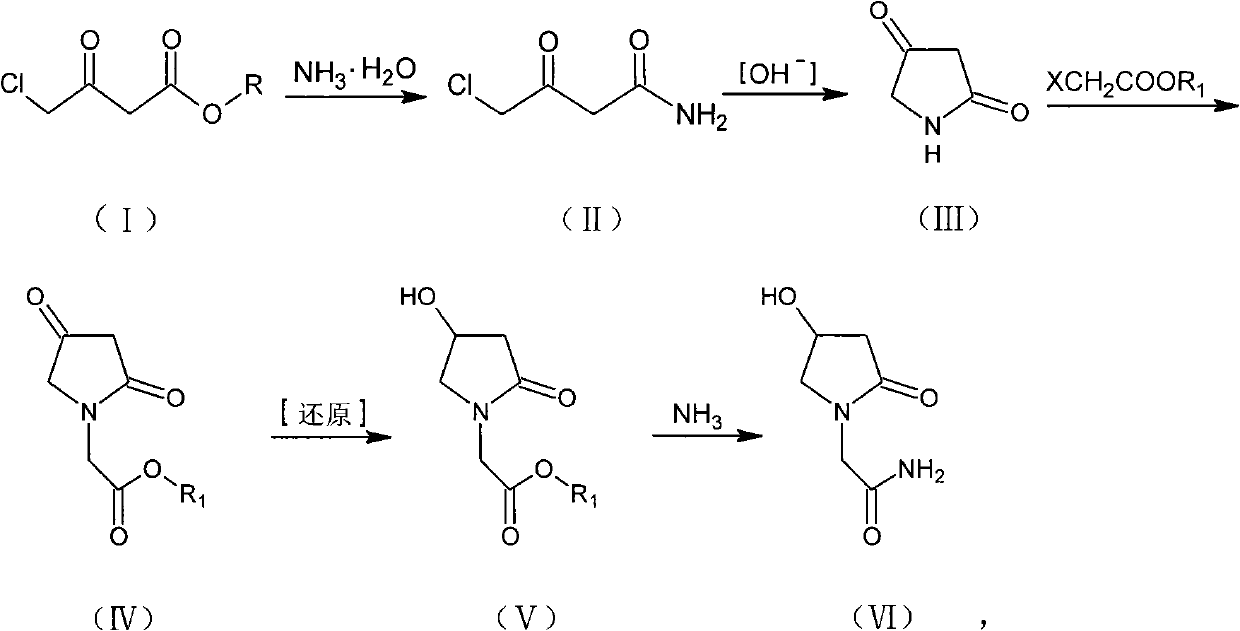
[0028] Embodiment 1: the preparation of 4-chloroacetoacetamide (II)
[0029] Method 1: Add methyl 4-chloroacetoacetate dropwise to 100L of concentrated ammonia water with a concentration of 25-28w% cooled to 0-5°C
[0030] (I) 20kg, continue to stir and react for 2 hours after dropping, filter, wash the filter cake with cold water lower than 10°C, and dry to obtain 13.6kg of 4-chloroacetoacetamide (II), with a yield of 75.6%.
[0031] Method 2: Add dropwise ethyl 4-chloroacetoacetate to 100L of concentrated ammonia water with a concentration of 25-28w% cooled to 0-5°C
[0032] (I) 20kg, continue to stir and react for 2 hours after dropping, filter, wash the filter cake with cold water below 10°C, and dry to obtain 11.9kg of 4-chloroacetoacetamide (II), with a yield of 72.1%.
Embodiment 2
[0033] Embodiment 2: the preparation of pyrrolidine-2,4-dione (III)
[0034] Method 1: Mix 13kg of 4-chloroacetoacetamide (II) with 104L of methanol and 19.9kg of potassium carbonate, pH9-10, and heat up and reflux for 12 hours. Filtrate while hot, and cool the filtrate to 0-5°C for crystallization for 4 hours. Filter, wash with methanol, and dry to obtain 5.9 kg of pyrrolidine-2,4-dione (III), with a yield of 62.1% and a melting point of 120-122°C.
[0035] Method 2: Mix 13kg of 4-chloroacetoacetamide (II) with 104L of ethanol and 19.9kg of potassium carbonate, pH9-10, heat up and reflux for 8 hours and filter while hot, and cool the filtrate to 0-5°C for crystallization for 4 hours. Filter, wash with ethanol, and dry to obtain 6.1 kg of pyrrolidine-2,4-dione (III), with a yield of 64.2% and a melting point of 120.5-122°C.
[0036]Method 3: Mix 13kg of 4-chloroacetoacetamide (II) with 130L of acetone and 19.9kg of potassium carbonate, pH 9-10, heat up and reflux for 12 hour...
Embodiment 3
[0041] Example 3: Preparation of 2-(2,4-dioxopyrrolidin-1-yl) acetate (IV)
[0042] Method 1: Suspend 60% 2.5kg of sodium hydride in 60L of toluene, slowly add 6kg of pyrrolidine-2,4-dione (III), stir and react at room temperature for 1 hour, then add 8.2kg of ethyl chloroacetate dropwise at room temperature, drop After heating up to 50-60°C for 8 hours, cool to room temperature, add 20 L of water to wash the toluene layer, repeat once, separate the toluene layer, dry, filter, and concentrate to obtain 2-(2,4-dioxopyrrolidine- 1-yl) ethyl acetate (IV) 8.9kg, yield 79.5%, melting point 88-91°C.
[0043] Method 2: Suspend 60% 2.5 kg of sodium hydride in 60 L of toluene, slowly add 6 kg of pyrrolidine-2,4-dione (III), stir and react at room temperature for 1 hour, then add 7.2 kg of methyl chloroacetate dropwise at room temperature, drop After heating up to 50-60°C for 8 hours, cool to room temperature, add 20 L of water to wash the toluene layer, repeat once, separate the tolue...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com